Abstract
ABCB4, which is specifically expressed on the canalicular membrane of hepatocytes, exports phosphatidylcholine (PC) into bile. Because SM depletion increases cellular PC content and stimulates PC and cholesterol efflux by ABCA1, a key transporter involved in generation of HDL, we predicted that SM depletion also stimulates PC efflux through ABCB4. To test this prediction, we compared the lipid efflux activity of ABCB4 and ABCA1 under SM depletion induced by two different types of inhibitors for SM synthesis, myriocin and (1R,3S)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide, in human embryonic kidney 293 and baby hamster kidney cells. Unexpectedly, SM depletion exerted opposite effects on ABCB4 and ABCA1, suppressing PC efflux through ABCB4 while stimulating efflux through ABCA1. Both ABCB4 and ABCA1 were recovered from Triton-X-100-soluble membranes, but ABCB4 was mainly recovered from CHAPS-insoluble SM-rich membranes, whereas ABCA1 was recovered from CHAPS-soluble membranes. These results suggest that a SM-rich membrane environment is required for ABCB4 to function. ABCB4 must have evolved to exert its maximum activity in the SM-rich membrane environment of the canalicular membrane, where it transports PC as the physiological substrate.
Keywords: ATP binding cassette protein; ATP binding cassette transporter A1; myriosin; cholesterol; high density lipoprotein; (1R,3S)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide; bile acid
ABCB4, which is specifically expressed in the canalicular membrane of hepatocytes, is essential for the secretion of phospholipids, especially phosphatidylcholine (PC), into bile (1). Phospholipid secretion into bile is important for the protection of the canalicular membrane against the strong detergent action of bile salts, which aid in absorption of cholesterol from food. Dysfunction of ABCB4 results in a lack of phospholipids and surplus of bile salts in primary bile, and this compositional imbalance causes damage to biliary canaliculi followed by chronic and progressive liver disease, such as intrahepatic cholestasis and low-phospholipid-associated cholelithiasis syndrome (2–5). ABCB4 shares quite high (86%) amino-acid sequence similarity (6) with multidrug resistance protein 1 (ABCB1), an ATP-dependent multidrug exporter (7). We have demonstrated that ABCB4 also functions as an ATP-dependent exporter by showing that PC and cholesterol are exported from cultured cells expressing the wild-type human ABCB4, but not from cells expressing an ATP-hydrolysis-deficient mutant of ABCB4, when bile salts are present in the medium (8).
ABCA1 exports excess cellular cholesterol and PC to the extracellular lipid acceptor apoA-I to generate HDL and plays a major role in cholesterol homeostasis and HDL metabolism. ABCG1, another member of the ABC proteins, is also involved in these processes. ABCG1 exports cholesterol and SM when HDL, but not apoA-I, is added to the medium as the lipid acceptor (9). We have shown that the decrease in cellular SM content in the plasma membrane reduces cholesterol export by ABCG1, and that the function of ABCG1 is dependent on SM (10, 11). By contrast, reduced cellular SM content stimulates apoA-I-dependent efflux of cholesterol and PC through ABCA1 (12). To analyze the effects of SM level on the functions of ABCA1 and ABCG1, we used the Chinese hamster ovary (CHO)-K1 mutant cell line LY-A (13), which harbors a missense mutation in the ceramide transfer protein (CERT). In this cell line, the total amount of choline phospholipids was not affected by the mutation in CERT, while SM content was reduced by 28–35% (12). This result suggested that the opposite effects on the functions of ABCA1 and ABCG1 might depend directly on changes in the amounts of their transport substrates, PC and SM. Alternatively, these results suggest that ABCA1 and ABCG1 may function in different membrane environments: SM-poor and SM-rich membrane domains, respectively.
On the plasma membrane, various mesoscale (10–100 nm) domains, such as lipid rafts (14), are believed to be dynamically organized and reorganized and are involved in various cellular functions such as signal transduction. Mesodomains containing relatively high levels of SM and cholesterol are resistant to extraction in cold nonionic detergent, such as Triton X-100 (15). Lipids loaded onto apoA-I by ABCA1 are derived from Triton-X-100-sensitive membrane domains, whereas lipids loaded onto HDL, the lipid acceptor for ABCG1, are derived from Triton-X-100-resistant membrane domains (16, 17). ABCB4 functions in Triton-X-100-soluble fractions rather than insoluble fractions (18).
Because both ABCB4 and ABCA1 export PC, it is reasonable to predict that ABCB4 and ABCA1 function in similar membrane mesodomains and respond to changes in lipid content in the same way. In other words, reduction in cellular SM content can be predicted to stimulate PC efflux through ABCB4, as in the case of ABCA1. To test this prediction, we compared the lipid efflux activity of ABCB4 and ABCA1 under SM depletion induced by inhibitors of SM synthesis in the human embryonic kidney (HEK) 293 and baby hamster kidney (BHK) cell lines. To our surprise, reduced SM content had the opposite effect on ABCB4 relative to our prediction.
MATERIALS AND METHODS
Materials
Monoclonal antibody C219, which recognizes human ABCB4, was purchased from Centocor. The rat anti-human ABCA1 monoclonal antibody KM3073 was generated against the first extracellular domain (amino acids 45–639) of human ABCA1, as described previously (19). Anti-green fluorescent protein (GFP) antibody was purchased from Santa Cruz Biotechnology. Recombinant apoA-I was prepared as reported previously (20). Sodium taurocholate (NaTC) was obtained from Wako Pure Chemicals Industries. Myriocin was purchased from Enzo Life Sciences. The CERT inhibitor (1R,3S)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide (HPA-12) and its inactive stereoisomer were chemically synthesized (21, 22). All other chemicals were purchased from Sigma-Aldrich, Life Technologies, Nacalai Tesque, Serva Feinbiochemica, and Dojindo.
Cell culture
Both HEK293 and BHK cells were grown in a humidified incubator with 5% CO2 at 37°C in DMEM supplemented with 10% heat-inactivated FBS. HEK293 cells stably expressing human ABCA1 fused with GFP at the C terminus were established as described (23). HEK293 cells stably expressing human ABCB4 were established as described (8). BHK/ABCA1 cells (24) were a kind gift from the late Dr. John Oram. BHK cells in which expression of ABCB4 can be induced by mifepristone were established in this study.
Generation of BHK cells expressing human ABCB4
BHK cells expressing human ABCB4 were generated using the mifepristone-inducible GeneSwitch system (Invitrogen). BHK cell line transfected with the pSwitch plasmid was generously provided by late Dr. Oram (25). Human ABCB4 cDNA was inserted into pGene/V5-HisA(blasticidin), in which original zeocin resistance gene was replaced by blasticidin resistance gene. Cells were transfected with pGene/V5-HisA(blasticidin)/ABCB4, and stably transfected cells were selected with 350 μg/ml of hygromycin and 5 μg/ml of blasticidin. BHK/ABCB4 cell line, in which ABCB4 expression was highly induced by mifepristone, was isolated. Control BHK/mock cell line was established by transfecting pGene/V5-HisA(blasticidin).
Measurement of SM content
Cells were washed with PBS and then air-dried. Then, membrane lipids were extracted with hexane/2-propanol (3:2), and SM content was determined as described (26).
Cellular SM and cholesterol/PC efflux assay
Cells were subcultured in poly-l-lysine-coated 6-well plates at a density of 5 × 105 cells per well in DMEM containing 10% FBS. After incubation for 24 h, cells were washed with DMEM and then incubated in DMEM containing 0.02% BSA containing an SM inhibitor and mifepristone in the presence of 1 mM NaTC or 5 μg/ml recombinant apoA-I. After incubation for 16 or 24 h, lipids in the medium was extracted with chloroform-methanol (2:1), and the amounts of cholesterol and PC were determined by colorimetric enzyme assays as described (27). NaTC (1 mM) treatment for 24 h did not show significant cytotoxicity to BHK/ABCA1 and BHK/ABCB4 cells even after myriocin treatment at 80 μM (in this study, we used myriocin at 20 or 40 μM) either in the absence or presence of mifepristone (supplementary Fig. 1). Cellular protein concentration was measured using a BCA protein assay kit (Pierce).
Preparation of detergent-soluble and detergent-insoluble membrane fractions
Cells were harvested, suspended in MES-buffered saline (protease inhibitor, 0.15 M NaCl, 25 mM MES, pH 6.5) containing 1% detergent. The suspension was kept on ice for 20 min and then centrifuged at 14,000 g for 20 min. The supernatant was removed and used as the detergent-soluble fraction. Precipitate (detergent-insoluble fraction) was resuspended by sonication in HEPES-buffered saline (protease inhibitor, 0.15 M NaCl, 25 mM HEPES, pH 7.4) containing 1% detergent.
OptiPrep gradient fractionation
Harvested BHK cells were suspended in Tris-NaCl-EDTA (TNE) buffer, and a single-cell suspension was generated by repeatedly drawing the sample through a 26G needle. After centrifuging at 860 g for 5 min, supernatant was transferred to an equal volume TNE/2% CHAPS buffer to yield a final concentration of 1% CHAPS. After incubation on ice for 30 min, the samples were adjusted with 60% iodixanol to a final concentration of 40% iodixanol. The mixture was overlaid with 30% iodixanol in TNE, and finally with TNE. The samples were centrifuged at 166,000 g for 4 h. Proteins in each fraction were precipitated with cold TCA.
Western blotting
Proteins were separated on 5–20% gradient SDS polyacrylamide gels (Atto) and immunodetected with the indicated antibodies. Blots were analyzed and quantitated using an LAS-3000 imaging system and software (Fujifilm).
LC-ESI/MS/MS
The LC-ESI/MS/MS analysis was performed on a Shimadzu Nexera UHPLC system (Shimadzu, Kyoto, Japan) coupled with QTRAP 4500 hybrid triple quadrupole linear ion trap mass spectrometer (AB SCIEX, Framingham, MA). Chromatographic separation was performed on an Acquity UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters, Milford, MA) maintained at 40°C using mobile phase A [water-methanol 50:50 (v/v) containing 10 mM ammonium acetate and 0.2% acetic acid] and mobile phase B [isopropanol-acetone 50:50 (v/v)] in a gradient program (0–20 min: 30% B→70% B; 20–24 min: 90% B; 24–28 min: 30% B) with a flow of 0.5 ml/min. A neutral loss scan of 74 Da in the negative ion mode was used for detecting PC and SM. The instrument parameters were as follows (arbitrary units if not specified): Curtain Gas = 10 psi; Collision Gas = 7; IonSpray Voltage = −4,500 V; Temperature = 700°C; Ion Source Gas 1 = 40 psi; Ion Source Gas 2 = 80 psi; Declustering Potential = −105 V; Entrance Potential = −10 V; Collision Energy = −32 V; Collision Cell Exit Potential = −19 V. Product ion analysis in the negative ion mode was performed to determine the fatty acid composition of each PC species. Quantification was performed by integration of the peak area of the extracted ion chromatograms for each phospholipid species. Although ion peaks from a triple quadrupole mass spectrometer do not allow for direct comparison between phospholipid species, SM and PC standards showed the comparable peak area under the experimental conditions used in this study (supplementary Fig. 8).
Cell viability assay
Cell viability was estimated by measuring the lactate dehydrogenase activity in media and total cells using a CytoTox 96 NonRadioactive Cytotoxicity Assay Kit (Promega).
Statistical analysis
All experiments were repeated at least twice. Each replication of quantitative experiments was performed in triplicate. Values are presented as means ± SE. The statistical significance of differences between mean values was analyzed using the nonpaired t-test. Multiple comparisons were performed using the Dunnett test following ANOVA. A value of P < 0.05 was considered statistically significant.
RESULTS
ABCB4-dependent PC efflux is suppressed by SM reduction in HEK293 cells, whereas ABCA1-dependent PC efflux is enhanced
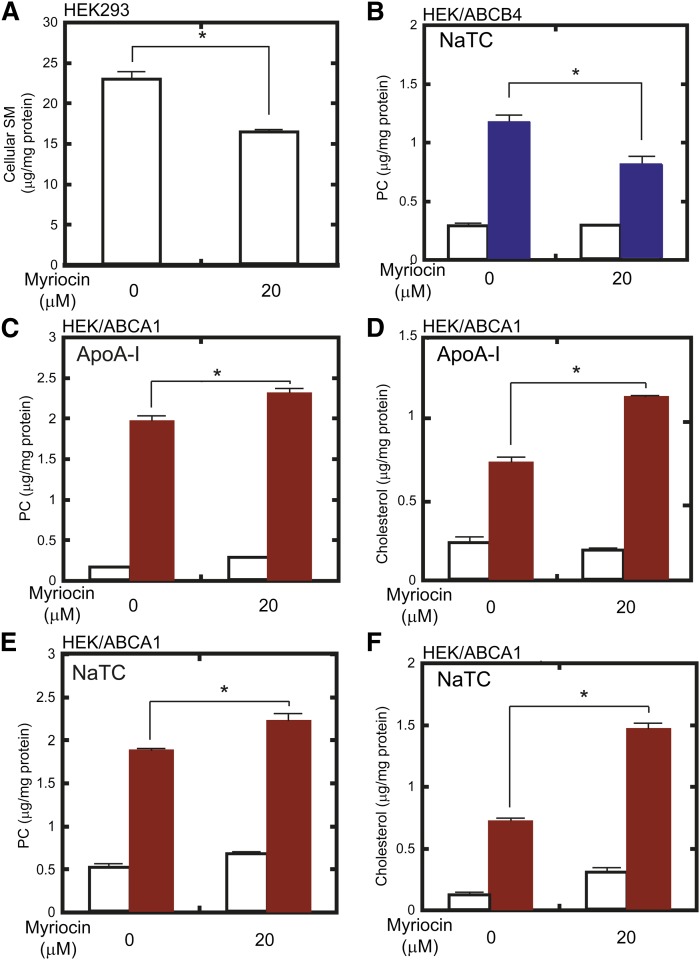
To examine the effect of SM depletion on the function of ABCB4, we reduced cellular SM content using myriocin, an inhibitor of sphingolipid synthesis (28). After a 24 h culture with 20 μM myriocin, SM content in HEK293 cells was reduced by 30% relative to untreated cells (Fig. 1A). In contrast to the case of ABCA1 (12), ABCB4-dependent PC efflux in the presence of 1 mM NaTC was reduced by 31% when the SM level was lowered (Fig. 1B).
Fig. 1.
Effect of SM depletion by myriocin on ABCB4- and ABCA1-dependent lipid efflux from HEK293 cells. A: HEK293 cells were incubated in the absence or presence of 20 μM myriocin for 24 h. Cellular lipids were extracted, and SM content was analyzed. B: HEK/ABCB4 cells were pretreated with 0 or 20 μM myriocin for 24 h. Then, cells were incubated in the absence (empty bars) or presence (blue bars) of 1 mM NaTC for 24 h. Lipids in the medium were extracted, and PC content was analyzed. C–F: HEK/ABCA1 cells were pretreated with 0 or 20 μM myriocin for 24 h. Then, cells were incubated in the absence (empty bars) or presence (red bars) of 10 μg/ml apoA-I (C, D) or 1 mM NaTC (E, F) for 24 h. Lipids in the medium were extracted, and PC (C, E) and cholesterol (D, F) contents were analyzed.
This result was contrary to our prediction that ABCB4 and ABCA1, which transport PC as their main substrate, would respond to changes in lipid content in similar ways. The previous observation of enhanced PC efflux through ABCA1 after SM depletion (12) was made in a mutant CHO cell line that has a defect in CERT (29). Therefore, we investigated whether a similar enhancement could be observed in HEK293 cells in which the SM content was reduced by myriocin treatment. Under the same conditions used in Fig. 1A, PC and cholesterol efflux through ABCA1 in the presence of apoA-I were enhanced by 18% and 37%, respectively (Fig. 1C, D). Whereas plasma apoA-I functions as a lipid acceptor for ABCA1 in vivo, NaTC can function as a lipid acceptor for ABCA1 in vitro (23). Therefore, we investigated whether enhanced lipid efflux through ABCA1 after SM depletion would also be observed in the presence of NaTC. As shown in Fig. 1E, F, PC and cholesterol efflux through ABCA1 were also enhanced in the presence of NaTC, by 18% and 109%, respectively. Myriocin treatment slightly increased ABC protein-independent release of PC and cholesterol from HEK293 host cells in the presence of NaTC or apoA-I (supplementary Fig. 2). But their differences were <0.2 μg/mg protein and marginal compared with those in ABC protein-dependent efflux (Fig. 1).
To confirm that the changes in PC and cholesterol efflux were not due to the changes of ABCB4 and ABCA1 expression, the effects of myriocin treatment on the amount of ABCB4 and ABCA1 were examined by Western blotting (supplementary Fig. 3). Myriocin treatment slightly but significantly increased the amount of ABCB4 in HEK293, while it did not increase the amount of ABCA1. These results further supported that myriocin treatment suppressed ABCB4-dependent PC efflux but enhanced ABCA1-dependent PC efflux. These results suggest that the lipid efflux activity of ABCB4 is suppressed and that of ABCA1 is enhanced when SM content is reduced, and that SM reduction induced by myriocin exerts opposite effects on the activity of ABCB4 and ABCA1.
SM depletion exerts opposite effects on the lipid-export activities of ABCB4 and ABCA1 in BHK cells
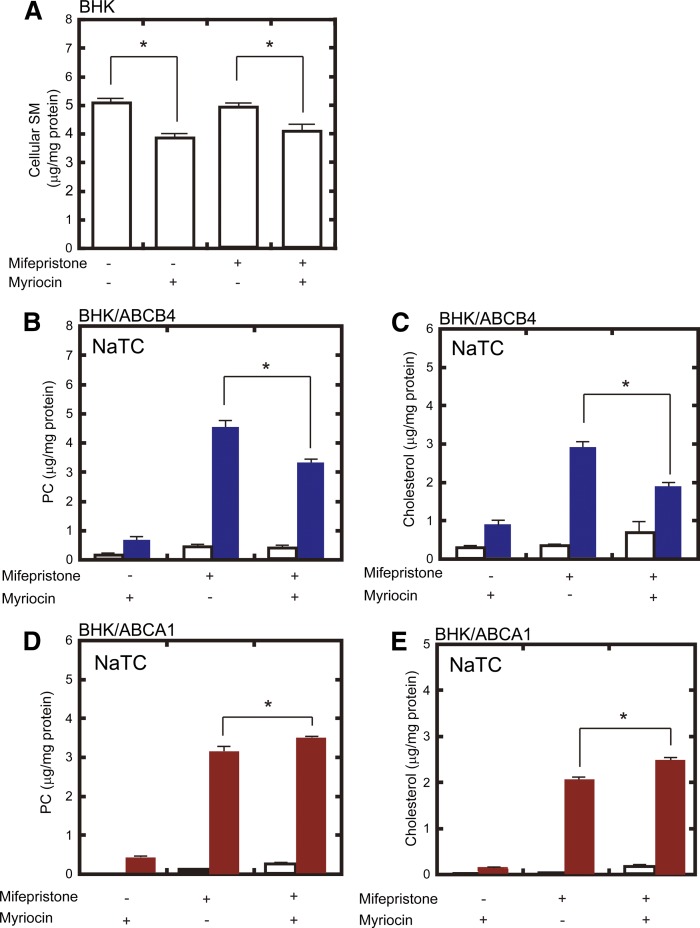
To confirm the results described above, we used BHK cells in which ABCA1 or ABCB4 expression can be induced by mifepristone, a synthetic steroid (supplementary Fig. 4). We also compared the activities of these proteins in the presence of the same lipid acceptor, NaTC. When BHK host cells were incubated with 40 μM myriocin for 24 h either before or after mifepristone treatment, cellular SM levels were reduced by 20–25% (Fig. 2A). When SM content was reduced, ABCB4-dependent PC and cholesterol efflux were lowered by 28% and 34%, respectively (Fig. 2B, C), whereas ABCA1-dependent PC and cholesterol efflux were enhanced by 11% and 24%, respectively (Fig. 2D, E). PC and cholesterol efflux by ABCA1 were also enhanced in the presence of apoA-I by 18% and 16%, respectively (supplementary Fig. 5). These results suggest that both ABCB4 and ABCA1 export PC and cholesterol, and that SM depletion exerts opposite effects on the lipid-export activities of ABCB4 and ABCA1.
Fig. 2.
Effect of SM depletion by myriocin on ABCB4- and ABCA1-dependent lipid efflux from BHK cells. A: BHK cells (empty bars) were incubated in the absence or presence of 40 μM myriocin and of 10 nM mifepristone for 24 h. Cellular lipids were extracted, and SM content was analyzed. B–E: BHK/ABCB4 cells (B, C) and BHK/ABCA1 cells (D, E) were pretreated with 0 or 40 μM myriocin and 0 or 10 nM mifepristone for 16 h. Then, cells were incubated in the absence (empty bars) or presence (filled bars) of 1 mM NaTC for 24 h. Lipids in the medium were extracted, and PC (B, D) and cholesterol (C, E) contents were analyzed.
HPA-12, another type of SM synthesis inhibitor, exerts the same effects as myriocin on ABCB4 and ABCA1
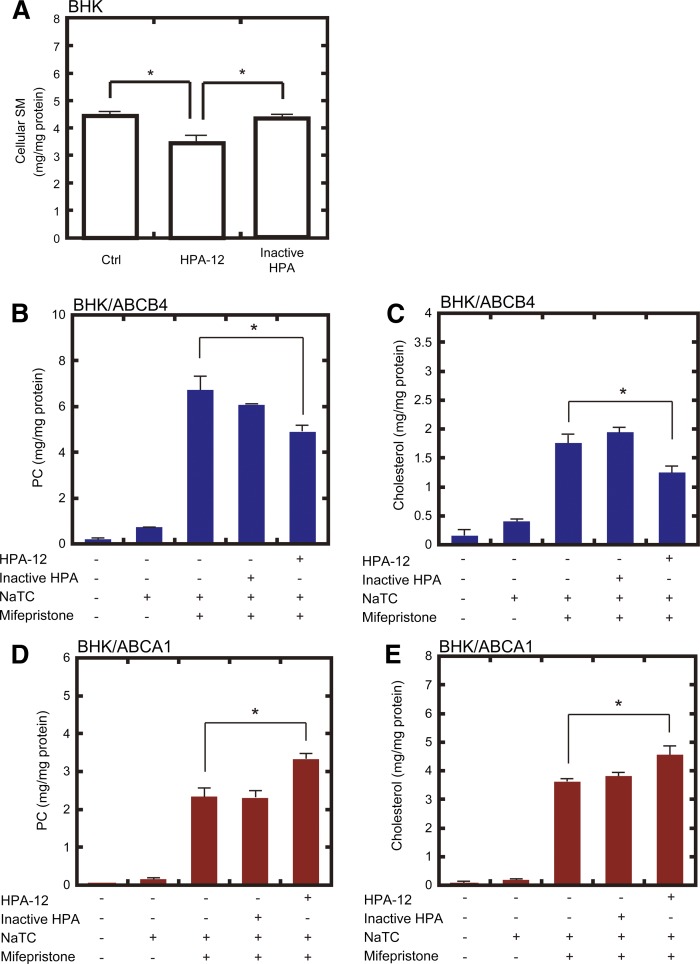
To confirm the effect of SM depletion, we utilized another type of SM synthesis inhibitor, HPA-12, which is a direct antagonist of ceramide transport protein (21, 22, 30, 31). After a 48 h culture with 10 μM HPA-12, SM content in BHK host cells was reduced by 25% relative to untreated cells (Fig. 3A). The inactive stereoisomers of HPA-12 did not affect SM levels. HPA-12 treatment reduced ABCB4-dependent PC and cholesterol efflux by 27% and 29%, respectively (Fig. 3B, C), whereas inactive stereoisomers had no significant effect. On the other hand, HPA-12 treatment enhanced ABCA1-dependent PC and cholesterol efflux by 42% and 26%, respectively (Fig. 3D, E). HPA-12 treatment slightly increased ABC protein-independent release of PC and cholesterol from BHK cells in the presence of NaTC or apoA-I (supplementary Fig. 6). But their differences were <0.2 μg/mg protein and marginal compared with those in ABC protein-dependent efflux (Fig. 3).
Fig. 3.
Effect of SM depletion by HPA-12 treatment on ABCB4- and ABCA1-dependent lipid efflux from BHK cells. A: BHK host cells were incubated in the presence of 10 μM HPA-12 or its inactive stereoisomer for 48 h. Cellular lipids were extracted, and SM content was analyzed. B–E: BHK/ABCB4 cells (B, C) and BHK/ABCA1 cells (D, E) were pretreated without or with 10 μM HPA-12 or its inactive stereoisomer and 0 or 10 nM mifepristone for 24 h. Then, cells were incubated in the presence of 1 mM NaTC for 24 h. Lipids in the medium were extracted, and PC (B, D) and cholesterol (C, E) contents were analyzed.
To confirm that the changes in PC and cholesterol efflux were not due to the changes in ABCB4 and ABCA1 expression, the effects of myriocin and HPA-12 treatment on the amount of ABCB4 and ABCA1 were examined by Western blotting (supplementary Fig. 7). Myriocin and HPA-12 treatment significantly increased the amount of ABCB4 in BHK cells, while they did not affect the amount of ABCA1. These results indicate that SM depletion suppresses the lipid-export activity of ABCB4, whereas it enhances that of ABCA1.
ABCB4 and ABCA1 are distributed differently in BHK cells
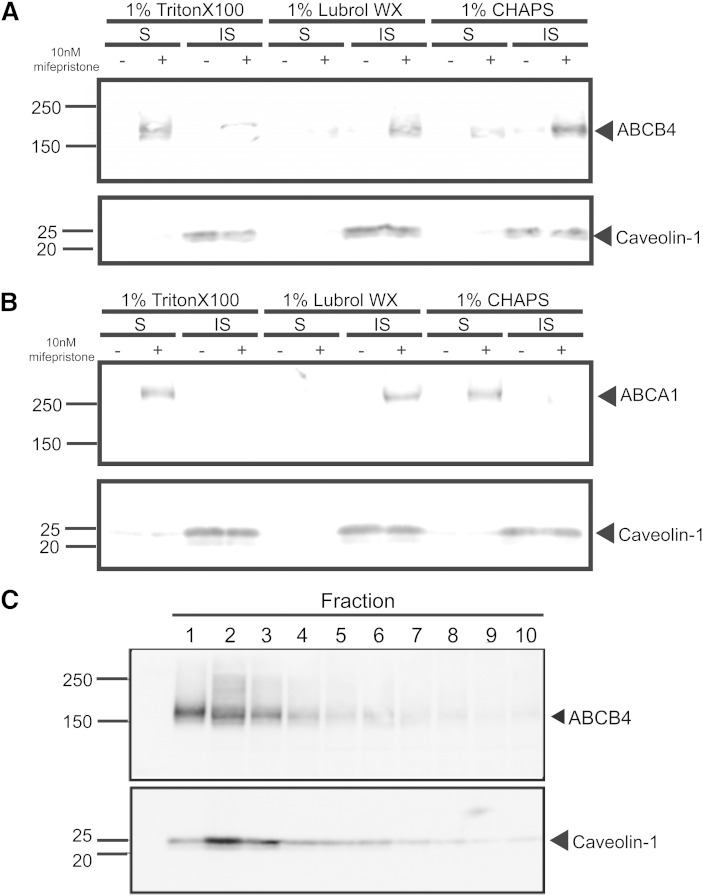
We speculated that the different effects of SM depletion on the lipid-export activities of ABCB4 and ABCA1 might be due to differences in their distributions on the plasma membrane. We examined these distributions using Triton X-100, Lubrol WX, and CHAPS. Both ABCB4 and ABCA1 were recovered from Triton-X-100-soluble membranes, as previously reported (12, 17, 18), as well as from Lubrol-WX-insoluble membranes (Fig. 4A, B). However, in the case of CHAPS, ABCB4 was mainly recovered from insoluble membranes, whereas ABCA1 was recovered from soluble membranes. In experiments with CHAPS, ABCB4 floated on the top of the OptiPrep gradient and was corecovered with caveolin-1 (Fig. 4C), indicating that ABCB4 mainly exists in CHAPS-resistant membrane fractions.
Fig. 4.
Different distribution of ABCB4 and ABCA1 in detergent-soluble and detergent-insoluble fractions. BHK/ABCB4 (A) and BHK/ABCA1 (B) cells were solubilized with 1% Triton X-100, 1% Lubrol WX, or 1% CHAPS. Proteins from the soluble (S) and insoluble (IS) fractions were separated on 5–20% gradient SDS polyacrylamide gels, and ABCB4 and ABCA1 were immunodetected. Caveolin was detected as a marker for detergent-insoluble fractions. OptiPrep gradient fractions were separated on 5–20% gradient SDS polyacrylamide gels. C: ABCB4 and caveolin-1 were detected with specific antibodies.
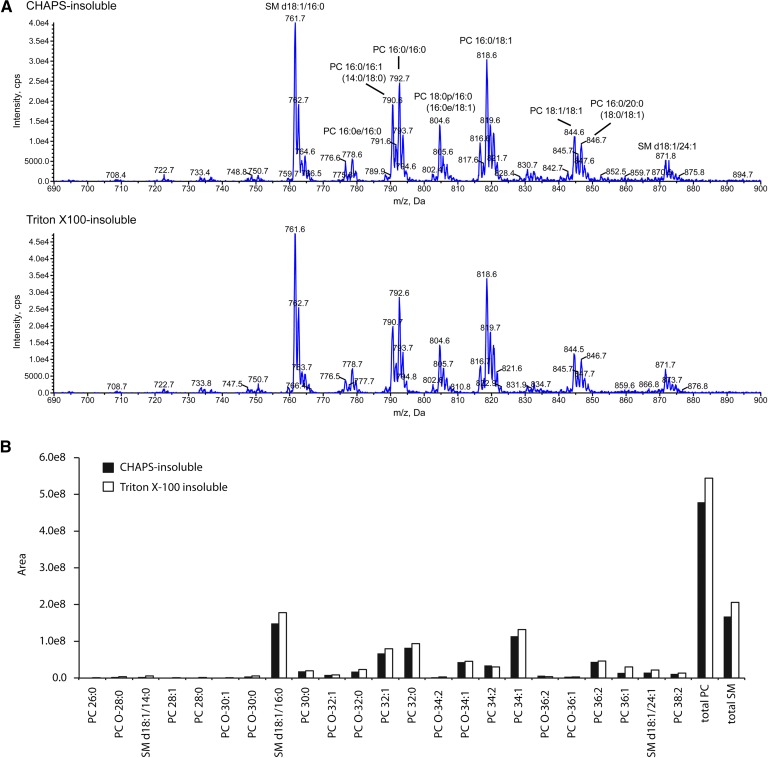
PC and SM species in CHAPS- and Triton-insoluble membrane fractions
When PC and SM contents were analyzed, PC/SM weight ratio in Triton-X-100-soluble and insoluble membranes were 15 ± 2 and 2.9 ± 0.5, respectively, and PC/SM ratio in CHAPS-soluble and insoluble membranes were 21 ± 5 and 4.5 ± 0.9, respectively (n = 15). PC/SM ratios were significantly different between soluble and insoluble fractions in both detergents; however, they were not between Triton-X-100 and CHAPS fractions. Therefore, we speculated that the different distribution of ABCB4 and ABCA1 in CHAPS- and Triton-insoluble membrane fractions might be due to different content of the PC and SM species (e.g., chain length and number of unsaturated bonds of fatty acids) in these membrane fractions. Then, PC and SM species were analyzed with MS (Fig. 5). Triton-X-100- and CHAPS-insoluble fractions contained various species of SM and PC, but SM and PC species and their relative amounts in these two fractions were not significantly different. These results suggest that the different distribution of ABCB4 and ABCA1 in CHAPS- and Triton-insoluble membrane fractions is due to other mechanisms, such as interacting with anchor proteins specifically distributed into these membrane fractions.
Fig. 5.
MS analysis of PC and SM species. A: Mass spectra of [M+CH3COO]− ions of PC and SM by scanning for neutral loss of 74 Da. Lipid extracts (0.7 nmol of total phospholipids) of CHAPS-insoluble and Triton-X-100-insoluble fractions were subjected to LC-ESI/MS/MS analysis. Assigning specific phospholipid species to m/z values was based on their calculated theoretical monoisotopic masses and verified by MS/MS. The prefix “d” represents sphingoid base, and the suffixes “e” and “p” indicate a chain with an alkyl ether linkage and a chain with a vinyl ether linkage, respectively. B: The amount of each molecular species of SM and PC was quantitated by integration of the peak area of the extracted ion chromatograms for the molecular species of SM and PC. PC molecular species are indicated by the total number of carbons and the number of double bonds in the fatty acyl chain. The prefix “O-” indicates an ether-type phospholipid.
DISCUSSION
Because both ABCB4 and ABCA1 export PC and cholesterol, we predicted that ABCB4 and ABCA1 function in similar membrane domains and respond to changes in lipid content in the same way. To our surprise, SM depletion exerted opposite effects on ABCB4 and ABCA1, suppressing the lipid-transport activity of ABCB4 but stimulating that of ABCA1.
To reduce cellular SM content, we used two types of inhibitors of SM synthesis. The biosynthesis of SM starts with the conjugation of l-serine and palmitoyl-CoA, catalyzed by serine palmitoyltransferase on the endoplasmic reticulum (ER) membrane. Myriocin blocks this first step of SM synthesis by reacting with serine palmitoyltransferase to form an adduct that resembles the natural intermediate of the catalytic reaction (13). Ceramide, the final intermediate synthesized on the ER membrane, is transferred via CERT (32) to the trans-Golgi where it is converted to SM. HPA-12, a synthetic analog of ceramide, interacts with CERT with high affinity and blocks SM synthesis but does not block glucosylceramide synthesis (21, 22, 30–33). Thus, when cells are treated with HPA-12, the content of SM can be reduced without a decrease of glycosphingolipid content. The reaction is highly specific, and stereoisomers of HPA-12 exert no effect on SM synthesis (21). We also used two cell lines to confirm the effects of these inhibitors. In HEK293 cells, ABCB4 and ABCA1 were stably expressed, whereas in BHK cells, expression of ABCB4 and ABCA1 was induced with a synthetic steroid using the Gene Switch system (34). We successfully reduced SM content in both HEK293 and BHK cells using myriocin and HPA-12 and examined the effects of these compounds on ABCB4 and ABCA1.
Various membrane proteins, including ABC proteins, localize in specific membrane domains, and the membrane environments affect protein functions. ABCB4 localizes in membrane domains soluble in Triton X-100 (18, 35), and ABCA1 is not recovered from either Triton-X-100- or Lubrol-WX-resistant membranes of fibroblasts (16). However, the relationship between protein activities and membrane environments remains unclear. We previously demonstrated that ABCA1 is distributed in SM-poor membrane domains, and SM depletion stimulates its PC efflux activity (12). We also reported that SM depletion suppresses ABCG1-dependent SM and cholesterol efflux (11). Because the previous results suggested that the total amount of choline phospholipids was not affected by the mutation in CERT when SM content was reduced (12), the opposite effects of SM depletion on the functions of ABCA1 and ABCG1 could be attributed to changes in the amounts of their transport substrates, PC and SM. Therefore, it is not possible to determine the relationship between protein activities and membrane environments based on the results obtained for ABCA1 and ABCG1. Here we demonstrated that SM depletion suppresses PC efflux function by ABCB4, whereas the main transport substrate for ABCB4 is PC. Therefore, the suppression of ABCB4 function by SM depletion cannot be attributed to a change in the level of the transport substrate.
ABCB4 was recovered from the CHAPS-insoluble membrane fraction, in which SM was concentrated compared with the CHAPS-soluble membrane. However, the PC/SM weight ratio was not significantly different between CHAPS-insoluble and Triton-X-100-insoluble membranes. Therefore, we speculated that the specific distribution of ABCB4 in the CHAPS-insoluble membrane might be due to specific PC and SM species (e.g., chain length and number of unsaturated bonds of fatty acids) in this fraction. However, MS analysis revealed that SM and PC species and their relative amounts in these two fractions were not significantly different (Fig. 5). The cause of the different distribution of ABCB4 and ABCA1 in different detergent-insoluble fractions is to be studied further.
The canalicular membrane is rich in SM and cholesterol relative to the basolateral membrane (36). These high levels of SM and cholesterol are probably necessary in order for bile canaliculi to tolerate high concentrations of bile salts, which are powerful detergents. Indeed, the results of extractions of rat canalicular membranes by bile salts suggest that bile-salt-resistant membrane domains exist in these membranes (37). These studies reported that at least two different types of detergent-resistant domains are present in the canalicular membranes of rat hepatocytes. ABCB4 is not present in Triton-X-100-resistant domains, but rather in other SM and cholesterol-rich membrane domains (35). However, bile contains PC as the predominant (90–95%) phospholipid and contains SM in only trace amounts (38). We previously showed by MS analysis that ABCB4 expressed in HEK293 cells preferentially mediated the efflux of PC over that of SM (8). These results indicate that PC is mainly transported by ABCB4 both in vivo and in vitro and that SM is not the substrate for ABCB4.
In summary, these results show for the first time that ABCB4 is distributed in an SM-rich membrane environment that is required for the protein’s function. Alternatively, SM may be required by ABCB4 as a cofactor. SM contains a free hydroxyl group, which may interact with proteins and cholesterol in the membrane. ABCB4 may have been evolved to be maximally active in the SM-rich membrane environment of canalicular membrane, where it transports PC as its physiological substrate.
Supplementary Material
Footnotes
Abbreviations:
- BHK
- baby hamster kidney
- CERT
- ceramide transfer protein
- CHO
- Chinese hamster ovary
- HEK
- human embryonic kidney
- HPA-12
- (1R,3S)-N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)dodecanamide
- NaTC
- sodium taurocholate
- PC
- phosphatidylcholine
- TNE
- Tris-NaCl-EDTA
This work was supported by a Grant-in-Aid for Scientific Research (S) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation. This work was supported by the World Premier International Research Center Initiative, MEXT.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures.
REFERENCES
- 1.Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A., et al. 1993. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 75: 451–462. [DOI] [PubMed] [Google Scholar]
- 2.Oude Elferink R. P., Paulusma C. C. 2007. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 453: 601–610. [DOI] [PubMed] [Google Scholar]
- 3.de Vree J. M., Jacquemin E., Sturm E., Cresteil D., Bosma P. J., Aten J., Deleuze J. F., Desrochers M., Burdelski M., Bernard O., et al. 1998. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA. 95: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleuze J. F., Jacquemin E., Dubuisson C., Cresteil D., Dumont M., Erlinger S., Bernard O., Hadchouel M. 1996. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 23: 904–908. [DOI] [PubMed] [Google Scholar]
- 5.Lucena J. F., Herrero J. I., Quiroga J., Sangro B., Garcia-Foncillas J., Zabalegui N., Sola J., Herraiz M., Medina J. F., Prieto J. 2003. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 124: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 6.van der Bliek A. M., Kooiman P. M., Schneider C., Borst P. 1988. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 71: 401–411. [DOI] [PubMed] [Google Scholar]
- 7.Ueda K., Cornwell M. M., Gottesman M. M., Pastan I., Roninson I. B., Ling V., Riordan J. R. 1986. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem. Biophys. Res. Commun. 141: 956–962. [DOI] [PubMed] [Google Scholar]
- 8.Morita S. Y., Kobayashi A., Takanezawa Y., Kioka N., Handa T., Arai H., Matsuo M., Ueda K. 2007. Bile salt-dependent efflux of cellular phospholipids mediated by ATP binding cassette protein B4. Hepatology. 46: 188–199. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A., Takanezawa Y., Hirata T., Shimizu Y., Misasa K., Kioka N., Arai H., Ueda K., Matsuo M. 2006. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 47: 1791–1802. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama H., Kimura Y., Kioka N., Matsuo M., Ueda K. 2013. ATPase activity of human ABCG1 is stimulated by cholesterol and sphingomyelin. J. Lipid Res. 54: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano O., Kobayashi A., Nagao K., Kumagai K., Kioka N., Hanada K., Ueda K., Matsuo M. 2007. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J. Lipid Res. 48: 2377–2384. [DOI] [PubMed] [Google Scholar]
- 12.Nagao K., Takahashi K., Hanada K., Kioka N., Matsuo M., Ueda K. 2007. Enhanced apoA-I-dependent cholesterol efflux by ABCA1 from sphingomyelin-deficient Chinese hamster ovary cells. J. Biol. Chem. 282: 14868–14874. [DOI] [PubMed] [Google Scholar]
- 13.Hanada K. 2003. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 1632: 16–30. [DOI] [PubMed] [Google Scholar]
- 14.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 15.Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA. 100: 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobnik W., Borsukova H., Bottcher A., Pfeiffer A., Liebisch G., Schutz G. J., Schindler H., Schmitz G. 2002. Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic. 3: 268–278. [DOI] [PubMed] [Google Scholar]
- 17.Mendez A. J., Lin G., Wade D. P., Lawn R. M., Oram J. F. 2001. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 276: 3158–3166. [DOI] [PubMed] [Google Scholar]
- 18.Morita S. Y., Tsuda T., Horikami M., Teraoka R., Kitagawa S., Terada T. 2013. Bile salt-stimulated phospholipid efflux mediated by ABCB4 localized in nonraft membranes. J. Lipid Res. 54: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hozoji M., Munehira Y., Ikeda Y., Makishima M., Matsuo M., Kioka N., Ueda K. 2008. Direct interaction of nuclear liver X receptor-beta with ABCA1 modulates cholesterol efflux. J. Biol. Chem. 283: 30057–30063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohta K., Shibui T., Morimoto Y., Iijima S., Kobayashi T. 1993. High level production of human proapo A-I by fed-batch culture of recombinant Escherichia coli. J. Ferment. Bioeng. 75: 155–157. [Google Scholar]
- 21.Yasuda S., Kitagawa H., Ueno M., Ishitani H., Fukasawa M., Nishijima M., Kobayashi S., Hanada K. 2001. A novel inhibitor of ceramide trafficking from the endoplasmic reticulum to the site of sphingomyelin synthesis. J. Biol. Chem. 276: 43994–44002. [additions and corrections: Yasuda , S. , H. Kitagawa , M. Ueno , H. Ishitani , M. Fukasawa , M. Nishijima , S. Kobayashi , and K. Hanada. 2001. J. Biol. Chem. 288 : 24162.]. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai K., Yasuda S., Okemoto K., Nishijima M., Kobayashi S., Hanada K. 2005. CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 280: 6488–6495. [DOI] [PubMed] [Google Scholar]
- 23.Nagao K., Zhao Y., Takahashi K., Kimura Y., Ueda K. 2009. Sodium taurocholate-dependent lipid efflux by ABCA1: effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J. Lipid Res. 50: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughan A. M., Oram J. F. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 25.Oram J. F., Vaughan A. M., Stocker R. 2001. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J. Biol. Chem. 276: 39898–39902. [DOI] [PubMed] [Google Scholar]
- 26.Hojjati M. R., Jiang X. C. 2006. Rapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatidylcholine. J. Lipid Res. 47: 673–676. [DOI] [PubMed] [Google Scholar]
- 27.Abe-Dohmae S., Suzuki S., Wada Y., Aburatani H., Vance D. E., Yokoyama S. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39: 11092–11099. [DOI] [PubMed] [Google Scholar]
- 28.Delgado A., Casas J., Llebaria A., Abad J. L., Fabrias G. 2006. Inhibitors of sphingolipid metabolism enzymes. Biochim. Biophys. Acta. 1758: 1957–1977. [DOI] [PubMed] [Google Scholar]
- 29.Hanada K., Hara T., Fukasawa M., Yamaji A., Umeda M., Nishijima M. 1998. Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin. Genetic and biochemical evidence for complex formation of the LCB1 protein with the LCB2 protein for serine palmitoyltransferase. J. Biol. Chem. 273: 33787–33794. [DOI] [PubMed] [Google Scholar]
- 30.Ueno K., Huang Y.-Y., Yamano A., Kobayashi S. 2013. Revised stereochemistry of ceramide-trafficking inhibitor HPA-12 by X-ray crystallography analysis. Org. Lett. 15: 2869–2871. [DOI] [PubMed] [Google Scholar]
- 31.Ďuriš A., Wiesenganger T., Moravčíková D., Baran P., Kožíšek J., Daïch A., Berkeš D. 2011. Expedient and practical synthesis of CERT-dependent ceramide trafficking inhibitor HPA-12 and its analogues. Org. Lett. 13: 1642–1645. [DOI] [PubMed] [Google Scholar]
- 32.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426: 803–809. [DOI] [PubMed] [Google Scholar]
- 33.Kudo N., Kumagai K., Matsubara R., Kobayashi S., Hanada K., Wakatsuki S., Kato R. 2010. Crystal structures of the CERT START domain with inhibitors provide insights into the mechanism of ceramide transfer. J. Mol. Biol. 396: 245–251. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., O’Malley B. W., Tsai S. Y. 1994. A regulatory system for use in gene transfer. Proc. Natl. Acad. Sci. USA. 91: 8180–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismair M. G., Häusler S., Stuermer C. A., Guyot C., Meier P. J., Roth J., Stieger B. 2009. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 49: 1673–1682. [DOI] [PubMed] [Google Scholar]
- 36.Meier P. J., Sztul E. S., Reuben A., Boyer J. L. 1984. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J. Cell Biol. 98: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyot C., Stieger B. 2011. Interaction of bile salts with rat canalicular membrane vesicles: evidence for bile salt resistant microdomains. J. Hepatol. 55: 1368–1376. [DOI] [PubMed] [Google Scholar]
- 38.Alvaro D., Angelico F., Attili A. F., Antonini R., Mazzarella B., Ginanni Corradini S., Gentile S., Bracci F., Angelico M. 1986. Plasma lipid lipoproteins and biliary lipid composition in female gallstone patients. Biomed. Biochim. Acta. 45: 761–768. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.