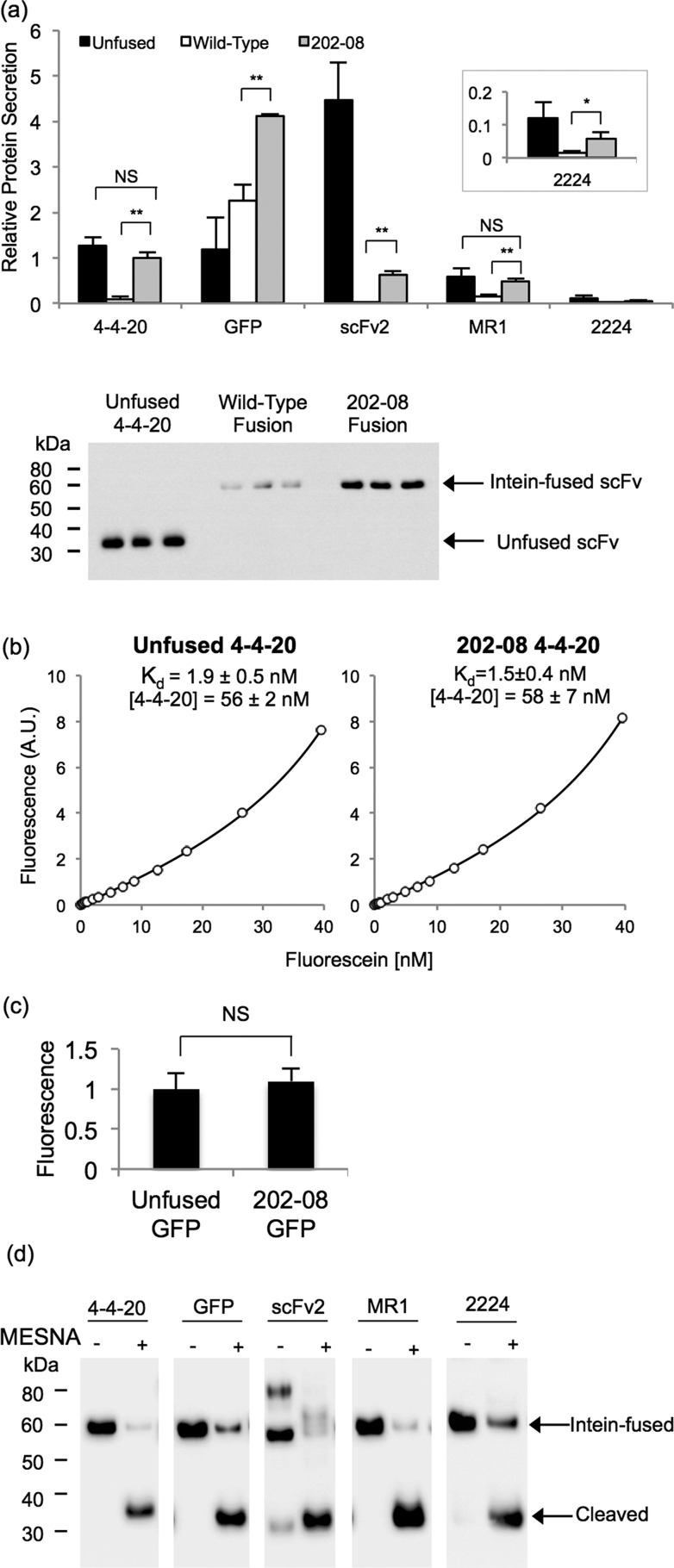
Figure 4.
Secretion of scFv and GFP intein fusion proteins. (a) Yeast supernatants containing scFv or GFP fused to the wild-type intein or 202–08 intein were subjected to anti-FLAG quantitative Western blotting and compared to the unfused target protein. Values are normalized to the level of the 4–4–20–202–08 fusion to determine relative amounts. The absolute secretion titer of the 4–4–20–202–08 fusion protein is 3.1 mg/L as determined in panel b. Reported are the means ± SD from three independent yeast transformants. Statistical significance was determined by an unpaired Student’s t-test (*p < 0.05; **p < 0.01; NS, not significant p > 0.05). Western blot of supernatant samples used for the quantitation of relative 4–4–20 protein secretion is shown below the bar graph. (b) An equilibrium binding curve was generated by fluorescein quenching to compare the Kd of unfused 4–4–20 and 4–4–20 fused to 202–08. A sample curve for each of the proteins is shown, and the mean ± SD for the fitted parameters of Kd value and 4–4–20 concentration were obtained by fitting quench curves generated from supernatants resulting from three independent yeast transformants. From the molar concentrations of 4–4–20, the average mass concentration of the 4–4–20 component was calculated to be 1.6 mg/L of yeast culture for both the unfused and the intein-fused 4–4–20 (corresponding to 3.1 mg/L for the full 4–4–20–202–08 fusion protein) The Kd and 4–4–20 concentrations were statistically indistinguishable, as determined by an unpaired Student’s t-test (p > 0.05). (c) GFP activity was determined by calculating the ratio of fluorescence to FLAG expression levels and normalizing to the unfused construct lacking intein. The mean ± SD results from three independent yeast transformants. The fluorescence per molecule of unfused GFP and 202–08 fused GFP was statistically indistinguishable, as determined by an unpaired Student’s t-test (**p > 0.05). (d) The catalytic activity of 202–08 was examined by reacting secreted and purified proteins with MESNA and evaluating cleaved yield after standard 20 h reaction. Anti-FLAG Western blotting demonstrates between 70% (2224) and 99% (MR1) release of the target protein from the 202–08 intein in the presence of MESNA.