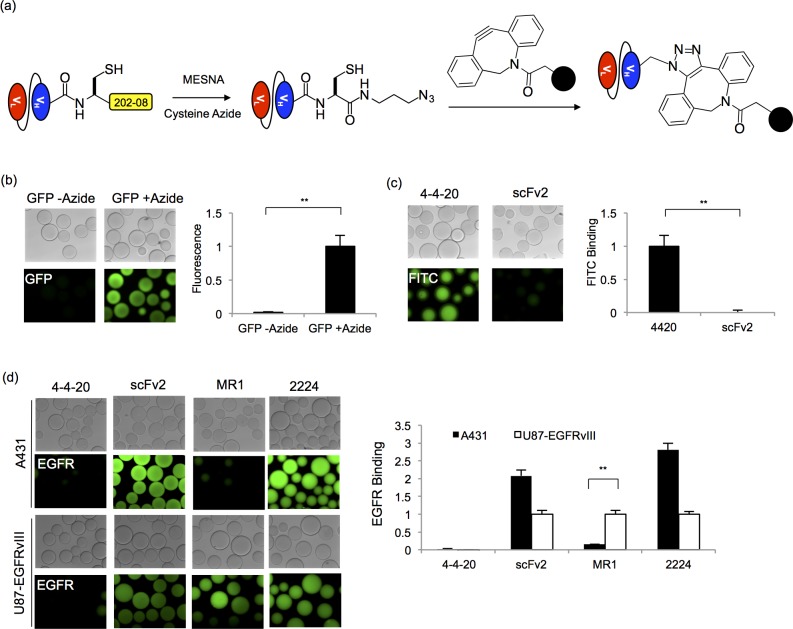
Figure 5.
Strain-promoted click chemistry immobilization. (a) Secreted and purified scFv and GFP proteins fused to the 202–08 intein were released with MESNA to form scFv- and GFP-thioesters. The carboxy-terminal thioesters were subsequently reacted with a cysteine azide via EPL to install an azido group onto the protein. To immobilize the proteins on surfaces, the scFv- and GFP-azide proteins were reacted with DBCO-functionalized agarose beads in a strain promoted click chemistry reaction. (b) Fluorescent microscope images of GFP fluorescence associated with beads reacted with GFP-azide or nonazido GFP (GFP-thioester). Relative protein immobilization was quantified by measuring total bead fluorescence and normalizing to the azide-GFP loaded beads. The mean ± SD of three independent immobilization reactions is plotted. Statistical significance was determined by an unpaired Student’s t-test (**p < 0.01) (c) Binding of fluorescein to beads reacted with azide functionalized 4–4–20 was analyzed and compared to beads reacted azide-linked scFv2. FITC-dextran binding was quantified by measuring the fluorescence intensity of the beads, and the fluorescence was normalized to the 4–4–20-linked sample. Three independent immobilization reactions were carried out to obtain the mean ± SD values. An unpaired Student’s t-test was performed to determine statistical significance (**p < 0.01) (d) Immobilized EGFR scFv activity was assessed by EGFR capture from cell lysates. Fluorescent microscopy images were employed to demonstrate EGFR capture and EGFR isoform specificity. A431 cells express wild-type EGFR while U87 cells are transfected to express the EGFR vIII isoform. ScFv activity was quantified by measuring the resulting fluorescence intensity of the beads, and the fluorescence value was normalized to the signal originating from the U87-EGFRvIII lysate binding to the respective scFv. The fluorescence value for the negative control, 4–4–20, was normalized to the signal originating from the U87-EGFRvIII binding to MR1. The mean ± SD of three independent immobilization reactions is plotted. Statistical significance was determined by an unpaired Student’s t-test (**p < 0.01).