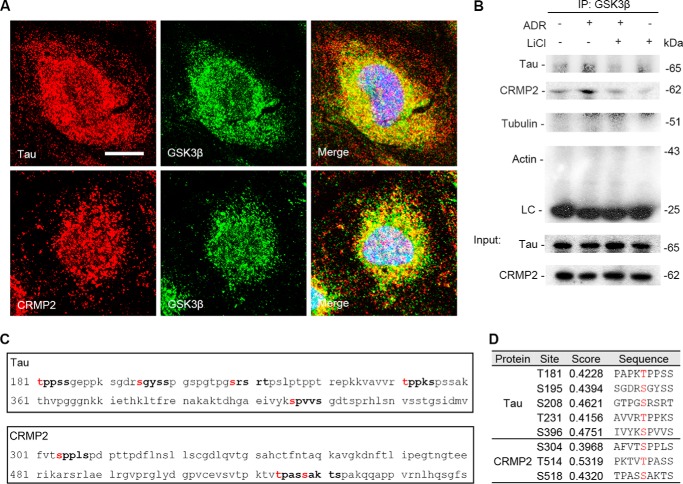
FIGURE 6.
Tau and CRMP2 colocalize and physically interact with GSK3β as its putative substrates in podocytes. A, representative micrographs of laser scanning confocal microscopy of dual color fluorescent immunocytochemistry staining of GSK3β and Tau or CRMP2 in differentiated conditionally immortalized mouse podocytes. Evident colocalization of Tau or CRMP2 with GSK3β was noted in the cytoplasm of differentiated podocytes. Bar, 5 μm. B, podocytes were treated as described in Fig. 2A, and cell lysates were prepared at 14 h for immunoprecipitation followed by immunoblot analysis for indicated molecules. LC, IgG light chain. An aliquot of cell lysates from each group served as input control. C, in silico analysis indicated that amino acid residues Thr-181, Ser-195, Ser-208, Thr-231, and Ser-396 of the longest isoform of small Tau and amino acid residues Ser-304, Thr-514, and Ser-518 of CRMP2 reside in the consensus motifs for phosphorylation by GSK3β. D, characteristics of consensus GSK3β phosphorylation motifs in Tau and CRMP2, including the predicted phosphorylation sites, prediction confidence scores, and sequences, as estimated by in silico analysis.