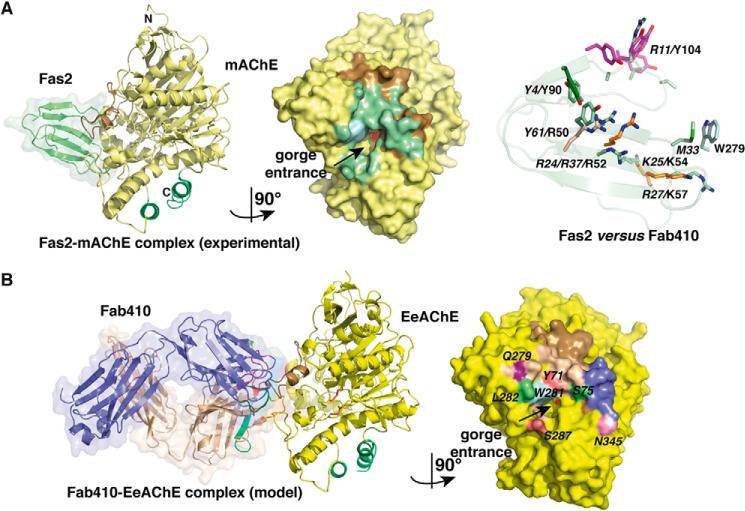
FIGURE 6.
Comparison of the Fab410-BfAChE complex with other peptidic inhibitor-AChE complexes. A, overall views of the crystalline Fas2-mAChE complex (left) (PDB code 1KU6) and the 1029-Å2 buried interface at the mAChE surface in this complex (center) (left and right mAChE molecules are oriented 90° from each other). Fas2 is displayed in light green, and mAChE Trp286 is in light blue (other color codes as in Fig. 3A). Right, spatial alignment of the key interacting aromatic and positively charged side chains in BfAChE-bound Fab410 (plain labels; color codes as in Fig. 5) and mAChE-bound Fas2 (italicized labels; green side chains) overlaid onto the Fas2 backbone (light green ribbon). Their similar positioning along with the absence of a Fab410 residue mimicking Fas2 Met33 in its interaction with Trp279 (displayed in light blue on the right) is evident. B, overall views of the theoretical model of the Fab410-EeAChE complex (left) and the 1017-Å2 buried interface at the EeAChE surface in this complex (right) (left and right EeAChE molecules are oriented 90° from each other). In the right panel, the buried EeAChE surface is colored according to the L chain and H chain CDRs in bound Fab410 (see Fig. 5A), and EeAChE Ser75, Leu282, and Asn345 are color-coded as in Fig. 5.