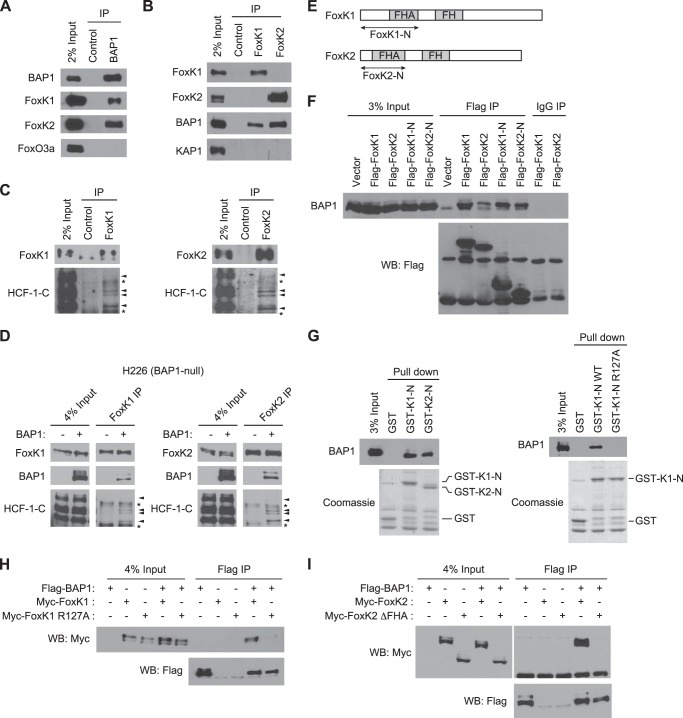
FIGURE 1.
FoxK1 and FoxK2 form ternary complexes with BAP1 and HCF-1. A, interaction of endogenous FoxK1/K2 with BAP1 in vivo. BAP1 was immunoprecipitated (IP) with specific antibodies, and the indicated proteins were detected by Western blotting. FoxO3a is shown as a negative control. B, association of FoxK1/K2 with BAP1 in vivo. FoxK1 or FoxK2 was immunoprecipitated, and the indicated proteins were detected by Western blotting. KAP1 is shown as a negative control. C, complex formation of FoxK1/K2 with HCF-1. FoxK1 or FoxK2 was immunoprecipitated, and the indicated proteins were detected by Western blotting. D, in vivo association of FoxK1/K2 with BAP1 and HCF-1 in H226 BAP1-deficient cells. BAP1 was expressed in H226 cells, and FoxK1 or FoxK2 was immunoprecipitated with specific antibodies. The indicated proteins were detected by Western blotting. The arrowheads and asterisks indicate HCF-1 bands and nonspecific bands, respectively. E, schematic of FoxK1 and FoxK2 proteins. F, interactions between the N-terminal regions of FoxK1/K2 and BAP1. Full-length or N-terminal regions (indicated in E) of FoxK1 and FoxK2 were expressed with a FLAG tag and immunoprecipitated with anti-FLAG antibodies or control IgG. Coprecipitated endogenous BAP1 was detected by Western blotting (WB). G, in vitro interactions between BAP1 and the N-terminal regions of FoxK1/K2. GST pulldown assays were performed with the indicated GST fusion proteins mixed with 293T cell lysates. GST fusion proteins in the precipitates were visualized by Coomassie staining, and coprecipitated BAP1 was detected by Western blotting. H, coimmunoprecipitation experiments of BAP1 and the FoxK1 R127A mutant. The indicated proteins were coexpressed in 293T cells, and FLAG-BAP1 was precipitated with anti-FLAG antibodies. Myc-FoxK1 was detected by anti-Myc Western blotting. I, coimmunoprecipitation experiments of BAP1 and the FHA deletion mutant (ΔFHA) of FoxK2.