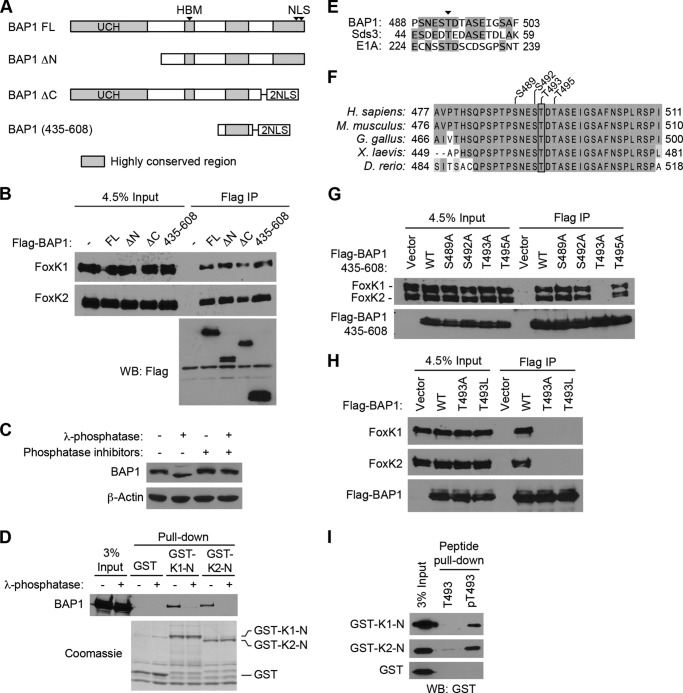
FIGURE 2.
Phosphorylation-dependent interactions between BAP1 and FoxK1/K2. A, schematic of BAP1 and its truncation mutants. Regions highly conserved between species are shown in gray. FL, full-length; UCH, ubiquitin C-terminal hydrolase; NLS, nuclear localization signal. B, mapping of the FoxK1/K2-interacting domains of BAP1. Full-length BAP1 and its truncation mutants, depicted in A, were expressed with a FLAG tag and precipitated with anti-FLAG antibodies. Coprecipitated endogenous FoxK1 and FoxK2 proteins were detected by Western blotting (WB). IP, immunoprecipitation. C, effect of λ-phosphatase treatments on BAP1 gel mobility. Cell lysates were treated with λ-phosphatase in the presence or absence of phosphatase inhibitors, and the indicated proteins were assessed by Western blotting. D, in vitro interaction between BAP1 and the N-terminal region of FoxK1/FoxK2. GST pulldown assays were performed using the indicated GST fusion proteins, and 293T cell lysates were treated with or λ-phosphatase or left untreated. Precipitated GST fusion proteins were visualized by Coomassie staining, and coprecipitated BAP1 was detected by Western blotting. E, alignment of BAP1, Sds3, and E1A sequences surrounding the conserved Thr residue (arrowhead), which is phosphorylated in Sds3. Identical amino acids are highlighted. F, alignment of the FoxK1/K2-interacting region of BAP1 from various species. Identical and similar amino acids are highlighted with different contrasts. G, in vivo interactions between BAP1 (435–608) and FoxK1/K2. FLAG-tagged BAP1 (435–608) with various point mutations was expressed in 293T cells and immunoprecipitated with anti-FLAG antibodies. Coprecipitated endogenous FoxK1 and FoxK2 were detected by Western blotting. H, in vivo interactions between FoxK1/K2 and full-length BAP1. FLAG-tagged BAP1 proteins (wild-type, T493A, and T493L) were expressed and immunoprecipitated with anti-FLAG antibodies. Coprecipitated endogenous FoxK1/K2 was detected by Western blotting. I, peptide pulldown assays. Peptides corresponding to the 487–499 amino acid region of BAP1 were synthesized with or without phosphorylation at Thr-493 and conjugated to beads. Pulldown assays were performed by mixing beads and recombinant GST-FoxK1-N or GST-FoxK2-N proteins, and precipitated GST-fusion proteins were detected by Western blotting using anti-GST antibodies. T493, non-phosphorylated peptide; pT493, peptide phosphorylated at Thr-493.