FIGURE 1.
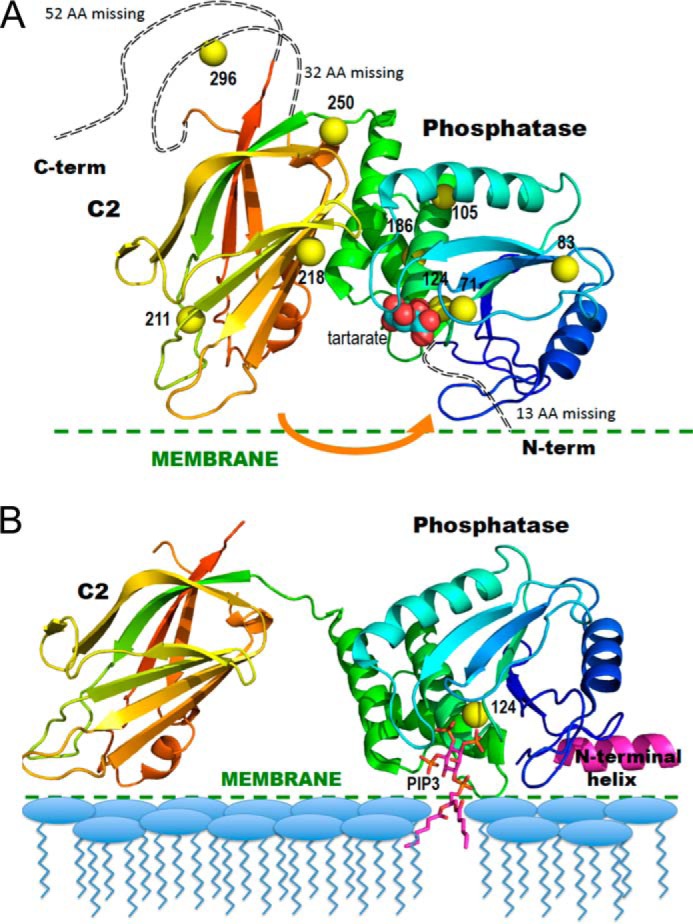
Ribbon diagrams of the crystal structure of PTEN in the absence and presence of substrate-containing membrane. A, PTEN crystal structure (Protein Data Bank code 1D5R) with colors from blue to red for N to C termini. Yellow spheres represent sulfur atoms of Cys residues that are spin-labeled in the NMR experiments. The tartrate molecule bound at the postulated active site is in van der Waals sphere representation. The arrow indicates a postulated movement of the two domains upon membrane binding. B, model for membrane-bound PTEN with the missing N-terminal peptide docked as an interfacial helix (pink). The sulfur of Cys-124 is depicted as a yellow sphere and a diC6PIP3 molecule (magenta) is docked in the active site. The postulated conformational change is based on that suggested by Kalli et al. (17).
