FIGURE 9.
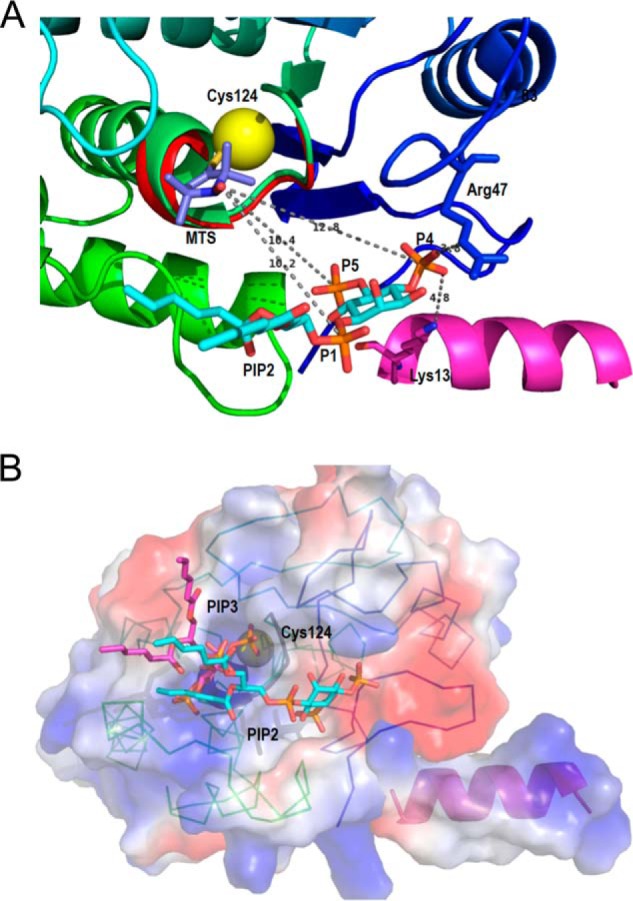
Ribbon diagrams of the model of diC6PI(4,5)P2 binding to PTEN at a discrete site near the active site are compatible with the 31P electron distances to the Cys-124 spin label. A, view of the phosphatase domain as seen from the membrane. The sulfur of Cys-124 is the yellow sphere to which the 2,2,5,5-tetramethyl-1-oxyl-3-methyl methanethiosulfonate is attached. The PI(4,5)P2 molecule, placed according to the surface features and to the relative distances determined by NMR, forms hydrogen-bonded contacts with Arg-47 and Lys-13, both critical residues for the PIP2 activation to be observed. B, surface representation of the phosphatase domain, in a similar view to that in A, colored by the electrostatic potential (red, negative, and blue, positive) with both substrate and activator docked to their sites. The PI(3,4,5)P3 molecule (magenta) is docked at the active site marked by the dark loop and the yellow sphere depicting Cys-124. The PI(4,5)P2 molecule (in cyan) occupies the same site as in A. The N-terminal helix is docked into the phosphatase domain, and its positive electrostatic potential complements the charges of the domain to create a positive membrane binding profile.
