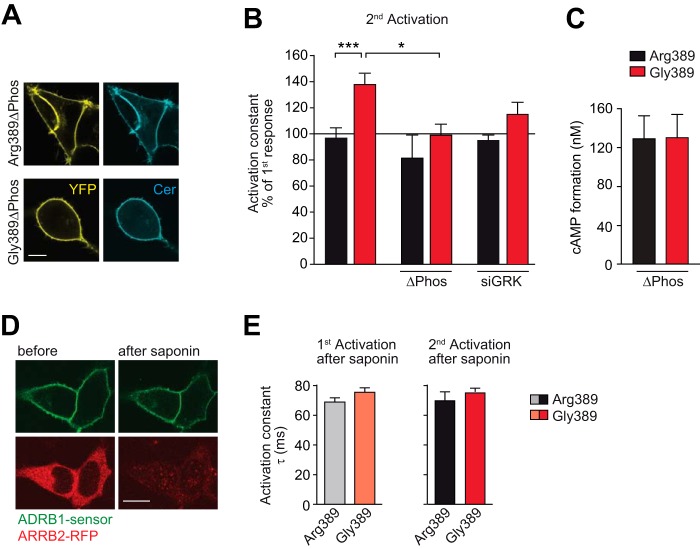
FIGURE 4.
The interaction of the activated ADRB1 with soluble intracellular proteins and ADRB1 phosphorylation is indispensable for variation-specific activation kinetics. A, cellular localization of the phosphorylation-deficient ADRB1 sensor mutants (ΔPhos). Scale bar = 10 μm. The mutations introduced to delete putative C-terminal phosphorylation sites are shown in Fig. 2D. B, activation kinetics without further intervention (data from Fig. 3D) upon mutation of C-terminal phosphorylation sites (ΔPhos) or cotransfection of an siRNA mixture targeting GRK-2, 3, 5, and 6 (n = 5–7). *, p < 0.05; ***, p < 0.001; two-way analysis of variance followed by Bonferroni post test. C, cAMP formation in HEK293 cells stably expressing the ΔPhos-ADRB1 sensor variants at comparable levels (Arg-389, 1.55 ± 0.22 pmol/mg; Gly-389, 1.49 ± 0.05 pmol/mg membrane protein; n = 3). Stimulation was carried out for 10 min with 1 μm NE in 384 wells containing 10,000 cells. D, qualitative control of saponin treatment. HEK293 cells expressing an ADRB1 FRET sensor at the plasma membrane and RFP-tagged β-arrestin 2 (ARRB2-RFP) in the cytosol before and after cell permeabilization with saponin for 3 min. Scale bar = 10 μm. E, activation kinetics of the ADRB1 sensors upon permeabilization-induced deprivation of intracellular proteins (n = 5). As in Fig. 3, the first stimulation was carried out for 5 min and the second stimulation directly after complete washout of the agonist.