FIGURE 3.
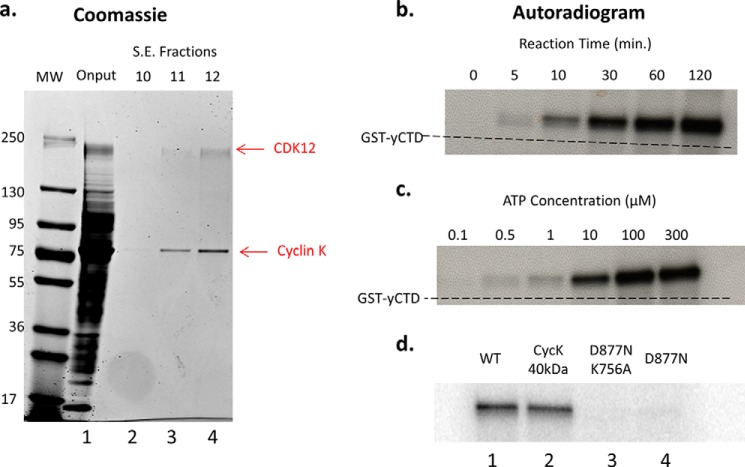
Purification and CTD kinase activity of hCDK12/CyclinK complex and mutants. a, Coomassie-stained gel of size exclusion chromatography (Superdex 200 HR 10/30) fractions from a hCDK12/CyclinK purification. Input (lane 1) consists of pooled Ni column elution fractions of hCDK12/CyclinK baculovirus-infected Sf9 cell nuclear extracts. The majority of fraction 10 (lane 2) consists of column void volume while fraction 11 (lane 3) is the first true elution fraction. b, purified hCDK12/CyclinK CTD kinase activity was assayed in the presence of [γ32-P]ATP using 1 μg of a GST-yCTD fusion protein (26 heptad repeats) as substrate and reaction mixtures were analyzed by SDS-PAGE. Autoradiograms of the SDS-PAGE gels revealed a time (ATP at 300 μm) and c, ATP-dependent (reaction time at 45 min) CTD kinase activity. The SDS-PAGE mobility of the unphosphorylated/unshifted GST-yCTD substrate is indicated via a dashed line. d, autoradiograms of CTD kinase assays using purified mutants of the hCDK12/CyclinK complex. Equal amounts of each complex are used in the reactions; hCDK12/CyclinK complex containing a shortened form of CyclinK exhibited comparable activity to full-length wild-type complex (compare lanes 1 and 2), while a kinase dead double (lane 3) and single mutant (lane 4) exhibited little to no residual activity.