Abstract
Although no species lives in isolation in nature, efforts to grow organisms for use in biotechnology have generally focused on a single-species approach, particularly where a product is required at high purity. In such scenarios, preventing the establishment of contaminants requires considerable effort that is economically justified. However, for some applications in biotechnology where the focus is on lower-margin biofuel production, axenic culture is not necessary, provided yields of the desired strain are unaffected by contaminants. In this article, we review what is known about interspecific interactions of natural algal communities, the dynamics of which are likely to parallel contamination in industrial systems. Furthermore, we discuss the opportunities to improve both yields and the stability of cultures by growing algae in multi-species consortia.
Introduction
Microalgae (eukaryotic photosynthetic microbes) and cyanobacteria (oxygenic photosynthetic bacteria) represent a highly diverse collection of microorganisms. They live in a range of environments including all aquatic ecosystems, both freshwater and marine, and are also found in terrestrial habitats including on hard surfaces and snow. Many taxa are capable of growing heterotrophically as well as phototrophically, and some obligate heterotrophs exist that, although ancestrally photosynthetic, have lost the ability to photosynthesize. These include the dinoflagellate Crypthecodinium cohnii, which is of commercial importance as a source of docosahexaenoic acid (DHA).1 Algae are currently cultivated on a relatively small scale for high-value products such as the carotenoid astaxanthin from Haematococcus pluvialis and the phycobiliprotein phycocyanin from the cyanobacterium Aphanizomenon flos-aquae.2 Certain strains are marketed as dietary supplements, including the cyanobacterium Spirulina sp. (Arthrospira platensis) and the green alga Chlorella vulgaris.
Bulk growth of algae for products of lower value—aimed at displacing commodities traditionally made from fossil oil—has received considerable attention in the research community in recent years.3 However, the scale-up required to achieve this poses a number of problems, ranging from the energy costs of maintaining the cultivation systems, low yields in large-scale cultures due to factors such as poor light penetration, mass transfer (where exogenous carbon dioxide is supplied, or oxygen needs to be removed), the energy costs of downstream processing and production, and biological contamination.4–6 This work considers how understanding the ecology of the organisms under cultivation—their interaction with others in the environment—can be harnessed to enhance productivity and increase the financial and environmental benefits of cultivating algae.
Applying Community Ecology to Algal Cultivation
Most studies that target increasing yields in industrial cultures are aimed at an individual species level, which assumes that cultivation will be in monoculture. However, because contamination is inevitable without stringent sterile practice, which at industrial scale is neither cost-effective nor likely to be achievable, understanding the growth dynamics of an algal population cultivated in reactors is fundamentally an ecological problem.7,8 Moreover, monocultures are by their nature unstable and prone to perturbation. According to a review by Smith and Crews, the genetic uniformity of monocultures encourages proliferation of pathogens and invaders, a problem common in traditional single-crop agriculture.9 Monocultures are predicted to be unstable by the basic tenets of community ecology, which describe natural systems as increasing in complexity over time.10 Given the opportunity, multiple species with diverse niche specificities can coexist alongside each other, maximizing the use of the available resources. Invasions by organisms from neighboring environments will continue until a “climax” stable state is reached; this state is predicted to be resilient to change provided abiotic conditions remain constant.11
Therefore, a strategy based on community approaches to cultivation is beginning to emerge. By starting with what would be an “end-point” consortium in a natural system, it may be possible to avoid the development of unwanted alternatives. In the following section we review the advantages—and disadvantages—of growing algae in consortia of species, rather than as monocultures. The principles that we draw on are from aquatic community ecology, the key concepts of which are summarized in Table 1.12–32
Table 1. Key Concepts from Aquatic Community Ecology and Freshwater Management.
| CONCEPTa | DESCRIPTION |
|---|---|
| Productivity- resource use complementarity | The productivity of a community is maximized when cohabitants have complementary resource requirements. Direct competition is decreased compared with a population of equal magnitude when members require the same resources.12–14 |
| Reciprocal exchange of nutrients | As part of a complex range of possible symbioses, many species have evolved to exchange metabolites that limit their growth in the environment. Such mutualisms strengthen the stability of the populations.15–18 |
| Regulation | Partners to a mutualism often regulate the growth of their symbionts in order to establish a fair (cost-efficient) exchange of nutrients.19,20 |
| Overyielding | Overyielding occurs when the net productivity of a community is greater than the average of the individual monocultures grown in the same environment. This is likely to be due to resource use complementarity, mutualisms, and enhanced stability against environmental perturbations.21,22 |
| PRACTICES FROM FRESHWATER MANAGEMENT/INDUSTRYb | DESCRIPTION |
|---|---|
| Biomanipulation | Biomanipulation is an ecosystem approach to freshwater management commonly aimed at reducing algal loads in lotic systems (such as lakes). Food web dynamics are used to shift community composition. For example, top predators may be introduced to reduce the density of zooplanktivorous fish, allowing the zooplankton grazers to decrease the algal load.23–25 |
| Interference grazing | Grazing zooplankton are able to consume certain algal species, dependent on their mouth size (known as ‘gape-limitation’). If a range of algal species of various sizes are grown together, grazers can be forced to spend more energy looking for prey that they can eat than they gain from feeding, which leads to their elimination from culture.26,27 |
| Probiotic control of unwanted bacteria | Bacteria are capable of eliminating other species from culture either directly or indirectly. Direct control is through the production of toxins that kill off competitors, while indirect control is through competition for resources. Once bacteria are present in the system, unwanted contaminants have a decreased likelihood of establishing.28,29 |
| Allelopathic control of contaminants | Allelopathy is a biological phenomenon that has been extensively documented in plant communities. Secondary metabolites that affect the survival, reproduction, and growth of neighboring species are released into the environment. Both positive and negative interactions have been recorded.30–32 |
Core ecological principles.
Main practices in freshwater management that have been used to direct communities towards a desired composition.
MAXIMIZING PRODUCTIVITY
One of the tenets of community ecology is that productivity is enhanced when diverse organisms are grown together. This has been illustrated for a range of habitats, and famously in a long-term experiment on grasslands. For a period of 7 years, it was demonstrated that grassland plots with a mixture of 16 species attained 2.7 times more biomass than the respective monocultures.12 “Overyielding” is said to occur when the biomass production of a consortium of species is greater than that of the average monoculture of the species contained in the mixture.21 While “transgressive overyielding” describes the state at which the mixture outperforms even the most productive of the monocultures of the constituent species.22 Evidence has shown that when functionally diverse algae are grown together, the resulting communities are more productive than monocultures of individual species. An aquatic experiment showed that diverse algal communities (grown in biofilms) increased the uptake and storage of nitrate from streams and significantly increased in biomass content compared to monocultures.33 Behl et al. analyzed the rate of carbon uptake and productivity for 85 assembled communities composed of species from four phylogenetic groups: chlorophytes, diatoms, cyanobacteria and chrysophytes.34 The researchers found that all algal communities consisting of species from two, three, or four different groups showed overyielding compared with cultures of individual species, with transgressive overyielding in more than half of the assemblages studied.
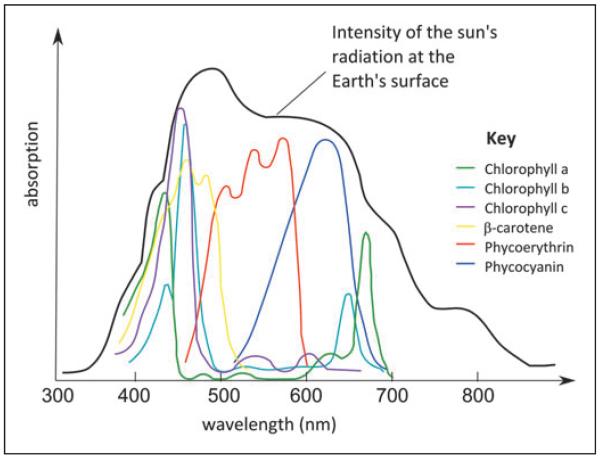
An important explanation for increased productivity in mixed cultures is through resource-use complementarity. When species that have different growth requirements are grown together, competition between members of the community is reduced compared with that experienced by individuals in dense monocultures. This allows more individuals to cohabit, increasing the net biomass of the culture. One trait that distinguishes different algal species is the portfolio of pigments they use to absorb light. Although oxygenic photosynthetic organisms generally use chlorophyll a as the major pigment in photosystems, the accessory light harvesting pigments differ (Fig. 1). In cyanobacteria grown under iron-replete conditions, phycobilisomes on the surface of the thylakoid membranes contain phycobilin proteins containing phycocyanin and phycoerythrin. Phycobilins are also found in red algae, whereas green algae (chlorophytes) contain chlorophyll b, as do all land plants. Chlorophyll c is the major accessory pigment in the Chromalveolata. Therefore, a possible explanation for the overyielding of biodiverse algal cultures observed by Behl et al. could be maximal use of the available light resource.34
Fig. 1.
Absorption spectra of pigments present in algae shown in comparison to the intensity of the sun’s radiation at the Earth’s surface. In vivo absorption spectra are shown for chlorophyll a (λmax = 435, 665 nm), chlorophyll b (λmax = 480, 650 nm), chlorophyll c (λmax = 645 nm), β-carotene (λmax = 450 nm), phycoerythrin (λmax = 490, 546, 576 nm), and phycocyanin (λmax = 618 nm).
CROP PROTECTION
Contaminating organisms that invade algal cultures can reduce yields in different ways: predators and pathogens are able to do so directly by killing the algae, while competing microalgae can take over as the dominant strain. For biomass production this may not be a problem, but when a specific algal strain is required, such as an oil producer or a strain with useful pigments, this will reduce productivity. In principle it could be possible to address all of these challenges by growing algae in culture with carefully selected cohabitating species.
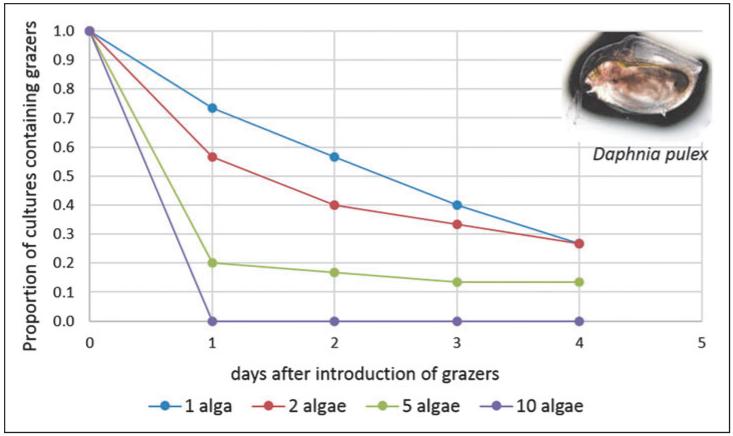
The effect of predators can be decreased through biomanipulation of the food web, whereby an ecosystem is deliberately altered by adding or removing species. This is common practice in the freshwater management industry, where the goal is to minimize microalgal production.35 In the context of algal cultivation, which is the reverse scenario, if production were to be hampered by invading zooplankton, the addition of zooplanktivores (such as small fish) to the reactors might increase yields.8,36 This is unlikely to be feasible in closed photobioreactors, but may also not be practicable in open ponds. An alternative solution would be crop protection through “interference” (Table 1). By introducing multiple inedible algal species to grow alongside the desired strain, the foraging efficiency of invading zooplankton may be decreased due to the increased energetic costs of finding the desired prey.37 This technique of pest control was recently investigated by Shurin et al. in a set of laboratory experiments, the results of which are summarized in Fig. 2.27 Communities containing one, two, five, or ten species of algae in various combinations were subjected to grazing by Daphnia pulex. Although the total biomass of algal food resources increased with diversity, survival of introduced Daphnia grazers declined markedly when five or ten species of algae were grown together.
Fig. 2.
Survival of Daphnia pulex (water flea) in cultures of algae with different species composition, taken from a study by Shurin et al.27 In this experiment, animals were added to 25-day-old cultures of algae composed of either one, two, five, or ten algal species (belonging to the groups Chlorophyta, Cyanophyta, Bacillariophyta and Heterokontophyta) and the number of cultures in which the fleas survived was assessed daily. For each combination, three biological repeats were assayed. The photograph is an image of Daphnia under the light microscope at 10× magnification.
However, there may be a cost to co-cultivation of a range of algal species when only a single species is of commercial interest. Since the growth of a desired strain may decrease in a dense polyculture due to increased shading by co-cultured strains. When stability of a monoculture against invasions is the primary concern, this may be enhanced by manipulating the abiotic environment to make the establishment of competitors less likely. This is why extremophiles have been preferred in commercial ventures, such as Spirulina sp., which is grown under alkaline conditions, or Dunaliella salina, which is cultured in highly saline medium.
A community solution to the problem of competitors may be engineered through co-culturing with partners that produce allelopathic chemicals. Chemical interactions are an important part of phytoplankton competition and are particularly important with dinoflagellates and cyanobacteria, which are able to produce chemicals that are toxic to most other algae in the environment.38 This allows the organisms to bloom under the right conditions for growth, often causing what are known as Harmful Algal Blooms (HABs).39 However, some species have evolved to withstand the toxins produced during HABs and are able to cohabit with the toxin-producing strains. If either HAB-forming or HAB-tolerant species were identified as interesting candidates for biofuel production, growth in consortia with toxin-producing strains could be a possible solution to competitive invasion. A similar approach has been taken in water treatment, where barley straw is often used to control populations of unwanted algae. Toxins produced from the straw liquor are known to inhibit the growth of some algae but not others.32
Bacterial contaminants often invade cultures of algae because they are able to scavenge algal exudates as a source of fixed carbon. If the bacteria compete with algae for other nutrients, they often overtake the growth of the microalgae, leading to the establishment of anoxic conditions.40–42 Bacterial fouling (surface growth) can be very severe in closed bioreactors, requiring these systems to be shut down and fully flushed before operation can resume. This leads to yield losses and has an associated financial burden. We have previously suggested that bacterial contamination may be decreased through co-culturing algae with symbiotic (probiotic) bacteria that enhance algal growth, because invading bacteria would be less likely to establish as the bacterial niche is already occupied.43 There is some empirical evidence from fish aquaculture that supports this theory. For example, Sharifah and Eguchi reported that Roseobacter clade bacteria that are symbiotic with Nannochloropsis oculata (grown commercially for fish food) successfully inhibited the growth of the fish pathogen Vibrio anguillarum.44
CAPITALIZING ON MUTUALISMS
Mutualisms can be of benefit in industrial biotechnology of microalgae in a range of ways. Mutualistic exchange of metabolites can replace external inputs of scarce or expensive resources. For example, half of all algae are known to require vitamin B12 (cobalamin) for growth, but this is only synthesized by prokaryotes.45 Model laboratory consortia have been described in which vitamin B12-dependent algae can obtain cobalamin from vitamin B12-synthesizing bacteria, in exchange for a source of fixed carbon, and, indeed, in the case of the Dinoroseobacter shibae partnership with its dinoflagellate host, vitamin B1 is also exchanged (Fig. 3A).36,43 If this system were to be employed industrially, the bacteria could replace exogenous addition of vitamins into the medium, reducing material and energy inputs into the culture. Other described mutualisms include the provision of iron via siderophores from bacteria to algae in exchange for fixed carbon.18
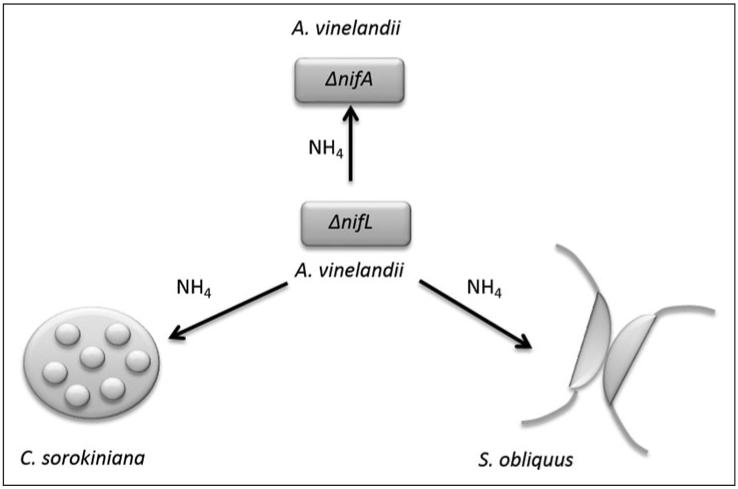
Fig. 3.
An engineered symbiotic interaction described by Ortiz-Marquez et al., in which a nitrogen-fixing bacterium, Azotobacter vinelandii, was modified to express constitutively a nitrogenase (ΔnifL) providing ammonium for non-diazotrophic organisms (unable to fix nitrogen), including the green algae Chlorella sorokiniana and Scenedesmus obliquus.47
It is possible to envisage a system in which the mutualism between algae and bacteria depends on provision of nitrogen by the bacteria, a macronutrient that is acknowledged as one of the key drivers of microalgal productivity in natural systems.48,49 Modelling the potential for algal biodiesel production in the United States indicated that the availability of nitrogen and phosphorus fertilizers were the major limiting factors for large-scale cultivation.50 In a recent study, Azotobacter vinelandii, a nitrogen-fixing bacterium, was genetically engineered to secrete ammonium into the surrounding medium.47 Co-culture of the strain in medium that did not contain exogenous carbon or nitrogen with oil-producing microalgae including Chlorella sorokiniana, Pseudokirchineriella sp. and Scenedesmus obliquus, allowed for growth of the algae (Fig. 3B).47 This shows the potential to grow algae industrially in the absence of nitrogenous fertilizer input by co-culturing with appropriate bacteria. As nitrogenous fertilizer is made through the energy-intensive Haber-Bosch process that has been estimated to contribute up to 40% of all energy inputs into microalgae biofuel systems, provision of nitrogen via a symbiont could significantly reduce the lifecycle energy and carbon footprint of the resulting fuel.51 Another possible sustainable approach would be to grow algae on wastewater that is rich in nitrogen and phosphorus, thereby recycling nutrients from domestic and agricultural effluent.52
It is likely that the range of options for co-culturing algae with bacteria will increase as our understanding of interspecific interactions between these organisms improves. Evidence suggests that microalgal interactions with bacteria are ubiquitous, although the physiological basis is often not known. For example, Park et al. reported that six out of the eight bacterial contaminants isolated from a Chlorella elipsoidea culture enhanced algal growth when co-inoculated with the species in a controlled co-culture.53 Similarly, Do Nascimento et al. found that the inoculation of Rhizobium strain 10II into cultures of the oleaginous microalga Ankistrodesmus sp. strain SP2-15 resulted in up to a 30% increase in accumulation of chlorophyll, biomass, and lipids compared with axenic monocultures of the alga.54
IMPROVING THE STABILITY OF DESIRED STRAINS
Regulation has been observed in the specific mutualism between the vitamin B12-dependent green alga Lobomonas rostrata and the soil bacterium Mesorhizobium loti, for which the ratio of algal-to-bacterial numbers equilibrated to around 1:30 in semi-continuous co-culture.43 Mathematical modelling of the dynamics of the two species in co-culture revealed that the population growth of one organism could be predicted entirely based on the expected carrying capacity of the co-cultured symbionts.55 Although the mechanism remains unknown, the biological implication is that the symbionts are controlling the the growth of one another when in co-culture. Understanding regulatory processes in symbioses may benefit biotechnology by providing a means to maintain the long-term stability of a co-culture and its fidelity.
It is well understood that bacterial genomes are highly dynamic and can adapt to selection pressures easily. Therefore, when proposing the engineering of an organism for a desired output, long-term stability of the genetic alteration should be an important consideration. An example of the highly dynamic nature of bacterial genomes is highlighted in a recent large-scale effort to re-sequence strains of wild type Synechocystis sp. PCC6803 (originally sequenced at University of California at Berkeley in 1971) that are being maintained in various culture collections around the world.56 The study revealed that strains presumed to be identical had in fact accumulated mutations likely to have effects on glucose tolerance, metabolism, motility, phage resistance, and stress responses.57,58 Likewise, genetically modified organisms are no less dynamic, and engineering the production and/or secretion of metabolites useful for biotechnology may create a significant energy burden. This burden can cause selection pressure to lose the introduced genes if there are no benefits in providing the service (i.e., only one organism is providing something useful). However, mutual exchange of essential metabolites may provide an environment whereby those genes are required to function in order to survive, thus mitigating against any selection pressure in favor of reversion. For example, Hosoda et al. engineered a syntrophic (cross-feeding) community of Escherichia coli in which two strains cohabited: one auxotrophic for isoleucine and the other for leucine.59 Neither strain was able to survive on its own, but growth was possible in synergistic co-culture. Kerner et al. engineered a similar system, in which E. coli were either tyrosine or tryptophan auxotrophs, but improved on the previous attempts by introducing an element of control to the system.60 By tuning the metabolic exchange via gene expression or chemical inducer they were able to regulate the growth rates and strain ratios. This was taken further to demonstrate successful interspecific associations, when an E. coli strain auxotrophic for glutamine was engineered to provide lipoic acid to Dictyostelium discoideum, an amoeba, in exchange for the amino acid.61
Towards Designing Algal Communities
We have identified four main advantages for using community approaches to cultivate microalgae. It is possible to increase productivity of microalgal cultures by cultivating consortia of species that have complementary functional traits and therefore overyield, or to decrease loss of productivity by cultivating microalgae with species from other life domains (such as non-photosynthetic bacteria or zooplanktivores), which can increase resistance to predators and contaminants. We have highlighted the importance of engineering co-dependence among introduced members of the consortium via mutualisms with the benefit of reducing energy and material inputs. Finally, in agreement with Brenner et al. we believe that for a stable and robust co-culture, whenever a new organism is introduced into a consortium it should contribute something useful to the culture “economy” in addition to receiving something in return, for example through the division of labor or specialization.62 In that way, interacting organisms rely on each other through trading to establish a stable and long-lasting culture.
Of course the use of consortia of microbes in biotechnology is not novel; multispecies systems are often employed to increase yields in microbial-based processes such as anaerobic digestion, fermentation, and bioremediation.63 In these traditional systems microbial communities are allowed to develop naturally; the most efficient assemblages are chosen for application and subsequently carefully maintained. Although this approach is not common in algal biotechnology, recently Mooij et al., demonstrated that by providing a selection pressure for algae to accumulate storage compounds linked directly to fitness, communities rich in starch and/or lipid assembled stochastically.64
These directed selection approaches will prove very useful to understanding the complex and advantageous interactions of microorganisms. In parallel to these efforts, we proposed a synthetic ecology approach to assembly of consortia aimed to be more productive and/or more resistant to contamination.36 Synthetic ecology differs from selection approaches by introducing an element of design and use of transferrable building blocks (such as specific species, engineered symbioses, or growth conditions) to assemble a desired community of microorganisms. However, all community approaches to cultivation remain unproven at scale. Indeed, the stability of an engineered consortium may face the same challenges as monocultures. A range of unanswered questions remains, the most fundamental of which is how much complexity within a consortium is required before challenges faced by monocultures (instability, invisibility, etc.) are overcome? The use of high-throughput sequencing in microbial ecology is revealing the highly complex and dynamic nature of communities, but these natural systems may well harbor considerable functional redundancy, which could be simplified for the benefit of biotechnological purposes.65
Acknowledgments
We are grateful to Jonathan Shurin (University of California, San Diego) and colleagues for sharing their data on Daphnia grazing (Fig. 1). Elena Kazamia acknowledges funding from the FP7 DEMA project (Reference number 309086). Anthony S. Riseley received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/ under Research Executive Agency grant agreement n° 317184. This paper reflects only the views of the authors, and the European Union is not liable for any use that may be made of the information contained therein.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
REFERENCES
- 1.Mendes A, Reis A, Vasconcelos R, et al. Crypthecodinium cohnii with emphasis on DHA production: A review. J Appl Phycol. 2008;21(2):199–214. [Google Scholar]
- 2.Benedetti S, Benvenuti F, Pagliarani S, et al. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004;75(19):2353–2362. doi: 10.1016/j.lfs.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Stephens E, Ross IL, King Z, et al. An economic and technical evaluation of microalgal biofuels. Nat Biotechnol. 2010;28(2):126–128. doi: 10.1038/nbt0210-126. [DOI] [PubMed] [Google Scholar]
- 4.Scott SA, Davey MP, Dennis JS, et al. Biodiesel from algae: Challenges and prospects. Curr Opin Biotechnol. 2010;21(3):277–286. doi: 10.1016/j.copbio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev. 2010;14(1):217–232. [Google Scholar]
- 6.Singh A, Olsen SI. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl Energ. 2011;88(10):3548–3555. [Google Scholar]
- 7.Letcher PM, Lopez S, Schmieder R, et al. Characterization of Amoeboaphelidium protococcarum, an algal parasite new to the cryptomycota isolated from an outdoor algal pond used for the production of biofuel. PLoS One. 2013;8(2):e56232. doi: 10.1371/journal.pone.0056232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith VH, Sturm BSM, Denoyelles FJ, Billings SA. The ecology of algal biodiesel production. Trends Ecol Evol. 2010;25(5):301–309. doi: 10.1016/j.tree.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Smith VH, Crews T. Applying ecological principles of crop cultivation in large-scale algal biomass production. Algal Res. 2013 doi:10.1016/j.algal.2013.11.005. [Google Scholar]
- 10.Elton CS. The Ecology of Invasions by Animals and Plants. University of Chicago Press; Chicago, IL: 1958. [Google Scholar]
- 11.May RM. Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature. 1977;269:471–477. [Google Scholar]
- 12.Tilman D, Reich PB, Knops J, et al. Diversity and productivity in a long-term grassland experiment. Science. 2001;294(5543):843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- 13.Van Ruijven J, Berendse F. Diversity-productivity relationships: Initial effects, long-term patterns, and underlying mechanisms. Proc Natl Acad Sci. 2004;102(3):695–700. doi: 10.1073/pnas.0407524102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corcoran AA, Boeing WJ. Biodiversity Increases the productivity and stability of phytoplankton communities. PLoS One. 2012;7(11):e49397. doi: 10.1371/journal.pone.0049397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boucher DH. Biology of mutualism: Ecology and evolution. Croom Helm; London: 1988. p. 388. [Google Scholar]
- 16.Stachowicz JJ. Mutualism, facilitation, and the structure of ecological communities. Bioscience. 2001;51(3):235. [Google Scholar]
- 17.Croft MT, Lawrence AD, Raux-Deery E, et al. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438(7064):90–93. doi: 10.1038/nature04056. [DOI] [PubMed] [Google Scholar]
- 18.Amin SA, Green DH, Hart MC, et al. Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc Natl Acad Sci. 2009;106(40):17071–17076. doi: 10.1073/pnas.0905512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith D, Douglas A. The biology of symbiosis. Edward Arnold; London: 1987. p. 302. [Google Scholar]
- 20.Holland JN, DeAngelis DL, Schultz ST. Evolutionary stability of mutualism: Interspecific population regulation as an evolutionarily stable strategy. Proc Biol Sci. 2004;271(1550):1807–18014. doi: 10.1098/rspb.2004.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hector A, Bazeley-White E, Loreau M, et al. Overyielding in grassland communities: Testing the sampling effect hypothesis with replicated biodiversity experiments. Ecol Lett. 2002;5(4):502–511. [Google Scholar]
- 22.Schmid B, Hector A, Saha P, Loreau M. Biodiversity effects and transgressive overyielding. J Plant Ecol. 2008;1(2):95–102. [Google Scholar]
- 23.Shapiro J, Lamarra V, Lynch M. Biomanipulation: An ecosystem approach to lake restoration. In: Brezonik PL, Fox JL, editors. Symposium on Water Quality Management and Biological Control. University of California; Gainesville, FL: 1975. pp. 85–96. [Google Scholar]
- 24.Hansson LA, Annadotter H, Bergman E, et al. Minireview: Biomanipulation as an application of food-chain theory: Constraints, synthesis, and recommendations for temperate lakes. Ecosystems. 1998;1(6):558–574. [Google Scholar]
- 25.Scheffer M, Hosper SH, Meijer ML, et al. Alternative equilibria in shallow lakes. Trends Ecol Evol. 1993;8(8):275–279. doi: 10.1016/0169-5347(93)90254-M. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert JJ. The effect of Daphnia interference on a natural rotifer and ciliate community: Short-term bottle experiments. Limnol Oceanogr. 1989;34(3):606–617. [Google Scholar]
- 27.Shurin JB, Abbott RL, Deal MS, et al. Industrial-strength ecology: Trade-offs and opportunities in algal biofuel production. Ecol Lett. 2013;16(11):1393–1404. doi: 10.1111/ele.12176. [DOI] [PubMed] [Google Scholar]
- 28.Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000;64(4):655–671. doi: 10.1128/mmbr.64.4.655-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaseeharan B, Ramasamy P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett Appl Microbiol. 2003;36(2):83–87. doi: 10.1046/j.1472-765x.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- 30.Rice EL. Allelopathy. Academic Press; New York: 1974. [Google Scholar]
- 31.Bais HP, Vepachedu R, Gilroy S, et al. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science. 2003;301(5638):1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 32.Ferrier MD, Butler BR, Terlizzi DE, Lacouture RV. The effects of barley straw (Hordeum vulgare) on the growth of freshwater algae. Bioresour Technol. 2005;96(16):1788–1795. doi: 10.1016/j.biortech.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale BJ. Biodiversity improves water quality through niche partitioning. Nature. 2011;472(7341):86–89. doi: 10.1038/nature09904. [DOI] [PubMed] [Google Scholar]
- 34.Behl S, Donval A, Stibor H. The relative importance of species diversity and functional group diversity on carbon uptake in phytoplankton communities. Limnol Oceanogr. 2011;56(2):683–694. [Google Scholar]
- 35.Perrow MR, Meijer M-L, Dawidowicz P, Coops H. Biomanipulation in shallow lakes: State of the art. Hydrobiologia. 1997;342-343:355–365. [Google Scholar]
- 36.Kazamia E, Aldridge DC, Smith AG. Synthetic ecology—A way forward for sustainable algal biofuel production? J Biotechnol. 2012;162(1):163–169. [Google Scholar]
- 37.Duffy JE. Biodiversity and ecosystem function: The consumer connection. Oikos. 2002;99(2):201–219. [Google Scholar]
- 38.Legrand C, Rengefors K, Fistarol GO, Granéli E. Allelopathy in phytoplankton—Biochemical, ecological and evolutionary aspects. Phycologia. 2003;42(4):406–419. [Google Scholar]
- 39.Heisler J, Glibert PM, Burkholder JM, et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae. 2008;8(1):3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currie DJ. Large-scale variability and interactions among phytoplankton, bacterioplankton, and phosphorus. Limnol Oceanogr. 1990;35(7):1437–1455. [Google Scholar]
- 41.Grover JP. Resource competition and community structure in aquatic microorganisms: Experimental studies of algae and bacteria along a gradient of organic carbon to inorganic phosphorus supply. J Plankton Res. 2000;22(8):1591–1610. [Google Scholar]
- 42.Daufresne T, Lacroix G, Benhaim D, Loreau M. Coexistence of algae and bacteria: A test of the carbon hypothesis. Aquatic Microb Ecol. 2008;53:323–332. [Google Scholar]
- 43.Kazamia E, Czesnick H, Nguyen VTT, et al. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol. 2012;14(6):1466–1476. doi: 10.1111/j.1462-2920.2012.02733.x. [DOI] [PubMed] [Google Scholar]
- 44.Sharifah EN, Eguchi M. The phytoplankton Nannochloropsis oculata enhances the ability of Roseobacter clade bacteria to inhibit the growth of fish pathogen Vibrio anguillarum. PLoS One. 2011;6(10):e26756. doi: 10.1371/journal.pone.0026756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warren MJ, Raux E, Schubert HE, et al. The biosynthesis of adenosylcobalamin (vitamin B12) Nat Prod Rep. 2002;19:390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 46.Wagner-Döbler I, Ballhausen B, Berger M, et al. The complete genome sequence of the algal symbiont Dinoroseobacter shibae: A hitchhiker’s guide to life in the sea. ISME J. 2010;4(1):61–77. doi: 10.1038/ismej.2009.94. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz-Marquez JCF, Do Nascimento M, Dublan M de LA, Curatti L. Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl Environ Microbiol. 2012;78(7):2345–2352. doi: 10.1128/AEM.06260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schindler DW, Hecky RE, Findlay DL, et al. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci. 2008;105(32):11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conley DJ, Paerl HW, Howarth RW, et al. Ecology. Controlling eutrophication: Nitrogen and phosphorus. Science. 2009;323(5917):1014–1015. doi: 10.1126/science.1167755. [DOI] [PubMed] [Google Scholar]
- 50.Pate R, Klise G, Wu B. Resource demand implications for US algae biofuels production scale-up. Appl Energ. 2011;88(10):3377–3388. [Google Scholar]
- 51.Clarens AF, Resurreccion EP, White MA, Colosi LM. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol. 2010;44(5):1813–1819. doi: 10.1021/es902838n. [DOI] [PubMed] [Google Scholar]
- 52.Pittman JK, Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol. 2011;102(1):17–25. doi: 10.1016/j.biortech.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 53.Park Y, Je K-W, Lee K, et al. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia. 2007;598(1):219–228. [Google Scholar]
- 54.Do Nascimento M, Dublan MDLA, Ortiz-Marquez JCF, Curatti L. High lipid productivity of an Ankistrodesmus-Rhizobium artificial consortium. Bioresour Technol. 2013;146:400–407. doi: 10.1016/j.biortech.2013.07.085. [DOI] [PubMed] [Google Scholar]
- 55.Grant MAA, Kazamia E, Cicuta P, Smith AGS. Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal-bacterial cocultures. ISME J. 2014 doi: 10.1038/ismej.2014.9. doi:10.1038/ismej.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanier RY, Kunlsawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanesaki Y, Shiwa Y, Tajima N, et al. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 2012;19(1):67–79. doi: 10.1093/dnares/dsr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trautmann D, Voss B, Wilde A, et al. Microevolution in cyanobacteria: Re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012;19(6):435–448. doi: 10.1093/dnares/dss024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosoda K, Suzuki S, Yamauchi Y, et al. Cooperative adaptation to establishment of a synthetic bacterial mutualism. PLoS One. 2011;6(2):e17105. doi: 10.1371/journal.pone.0017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerner A, Park J, Williams A, Lin XN. A programmable Escherichia coli consortium via tunable symbiosis. PLoS One. 2012;7(3):e34032. doi: 10.1371/journal.pone.0034032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubo I, Hosoda K, Suzuki S, et al. Construction of bacteria-eukaryote synthetic mutualism. Biosystems. 2013;113(2):66–71. doi: 10.1016/j.biosystems.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Brenner K, You L, Arnold FH. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008;26(9):483–489. doi: 10.1016/j.tibtech.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Sabra W, Dietz D, Tjahjasari D, Zeng AP. Biosystems analysis and engineering of microbial consortia for industrial biotechnology. Eng Life Sci. 2010;10(5):407–421. [Google Scholar]
- 64.Mooij PR, Stouten GR, Tamis J, et al. Survival of the fattest. Energ Environ Sci. 2013;6(12):3404. [Google Scholar]
- 65.Hill JE, Seipp RP, Betts M, et al. Extensive profiling of a complex microbial community by high-throughput sequencing. Appl Environ Microbiol. 2002;68(6):3055–3066. doi: 10.1128/AEM.68.6.3055-3066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]