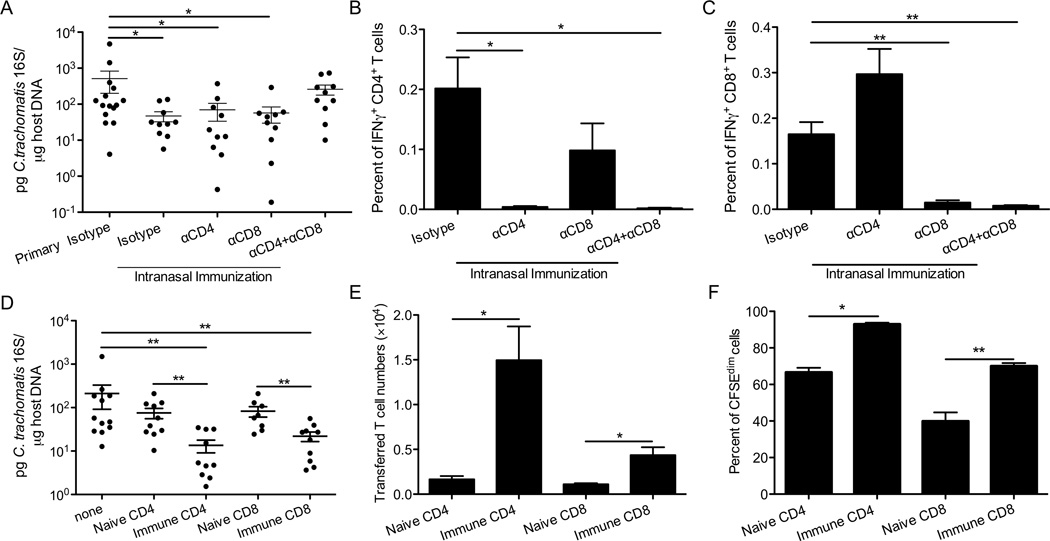
Figure 4.
T cells are required and sufficient for cross-mucosal protection against C. trachomatis. Groups of 10–15 C57BL/6 mice were intranasally immunized with C. trachomatis, rested for 4 weeks, and then treated with the neutralizing or isotype control antibodies as indicated. These mice and naïve mice that received isotype control antibodies (primary isotype) were then challenged in the genital tract. Six days after challenge, (A) bacterial burden was determined by quantitative PCR. (B) The percent of IFNγ+CD4+CD3+ or (C) IFNγ+CD8+CD3+ T cells among live cells from uterine tissues was determined by intracellular staining and flow cytometry. (D-F) CD90.1+ mice were immunized intranasally with C. trachomatis. A month later, CD4+ or CD8+ T cells were purified from SLOs of these mice or naïve control mice, labeled with CFSE, and transferred into groups of 8–12 CD90.2+ mice. These mice and mice that did not receive donor cells (none) were then challenged in the genital tract. Five days after challenge, (D) bacterial burden was determined by quantitative PCR. (E) Absolute number of donor cells and (F) percent of donor cells that diluted CFSE were determined by flow cytometry. These data are representative of at least 3 independent experiments. *: p ≤ 0.05. **: p ≤ 0.01.