Abstract
Microbial colonization of the infant occurs during a critical time window for immune and gastrointestinal development. Infant colonization sets the stage for the adult microbiome. This review is a broad survey of the factors affecting infant colonization and the downstream effects on gastrointestinal health and disease. Major topics affecting colonization include initial inoculation dependent on birth mode, the impact of breastfeeding, and inside-out modulation of the developing microbiome by the immune system. Major outcomes of colonization include the timing-dependent education of the neonatal immune system, which is interconnected with barrier function and metabolism. These all engage in further continuing cross-talk with the microbiome, genetics and nutrition. This review will also briefly discuss mechanisms of disease resulting from disrupted colonization as well as nutritional and microbial therapies.
Keywords: microbiome, infant colonization, breastfeeding, cesarean section, immune programming
Introduction
Initial colonization of the infant gut by microbes sets the stage for the lifelong, relatively stable adult microbiome1. Infants rely on colonization in order to complete development of the immune system and gastrointestinal tract. The first days and weeks of life represent a crucial window of opportunity for shaping the development of the gastrointestinal tract and immune system, as well as the adult microbiome. The nature of infant environmental exposures, acting through the microbiome, affect the likelihood of developing childhood and adult diseases such as obesity, food allergy, and inflammatory bowel disease.
NORMAL COLONIZATION AND ITS IMPACT ON HUMAN DEVELOPMENT
In cases of vaginal birth, the infant is inoculated as he or she passes through the birth canal. This inoculum is a mixture of gram negative and gram positive bacteria, aerobes and anaerobes2. At birth, the infant gut is an aerobic environment, which gradually becomes anaerobic over a period of days3,4. The earliest colonizing bacteria, facultative aerobes including Escherichia and Enterococcus, eventually establish an anaerobic environment. This enables the shift to obligate anaerobes, including Firmicutes such as Clostridia, Bacteroidetes, and especially Bifidobacteria5. Bifidobacteria comprise the largest group within the infant microbiome4,6. Throughout this succession of organisms, the microbiota increase in diversity6,7.
These initial colonizing species have come to be recognized as a pioneer microbiome, one which educates the developing immune system and provides favorable conditions for colonization by subsequent microbes, through production of an anaerobic environment, favorable substrates for bacterial growth, and protection from the systemic immune system4. This pioneer microbiome also has lifelong implications: bacterial strains sampled from adults are often shared between siblings and parents, demonstrating that childhood colonization can persist throughout adulthood8.
Beneficial factors that influence colonization
Many bacterial sources for the infant derive from the maternal microbiota. Therefore, beneficial infant colonization is dependent upon maternal genetics, environmental exposures and diet before and during pregnancy as well as during breast feeding. For example, prenatal maternal exposure to farm animals and consumption of raw milk is associated with decreased atopic disease9.
Increasing evidence demonstrates that the intrauterine environment is not sterile during pregnancy, contrary to prior dogma10. Bacteria have been cultured from meconium, umbilical cord blood, fetal membranes and amniotic fluid. Meconium, the infant’s first stool, is thought to reflect the pre-delivery intrauterine environment. Indeed, meconium colonization by several gut bacteria has been associated with infants born before 33 weeks, suggesting a pathologic role of intrauterine bacteria causing inflammation and subsequent preterm delivery11. Intrauterine colonization has also been associated with worse neonatal outcomes12. However, little is known of the mechanisms that allow for antenatal colonization of the infant. Given this gap in knowledge, as well as an absence of actionable steps to optimize colonization or to treat dysbiosis, this review will not delve deeply into this topic.
Delivery
The ideal infant intestinal colonization begins with oral inoculation by maternal vaginal microbiota. For a vaginally-delivered newborn, the intestinal microbiota closely resembles the microbiota of the mother’s vagina2. During vaginal birth, the infant is also inoculated with maternal intestinal bacteria13. In addition, maternal breast milk contains viable bacteria, sampled from the maternal gut, which serves as another source of bacterial diversity for the breastfeeding infant14.
Once the infant has been inoculated, compounds already present in the infant gut as well as from breast milk act as prebiotics and encourage growth of commensals. Prebiotics are compounds that are typically indigestible by human enzymes and instead serve as nutrition for the growth of microbes.
Natural prebiotics
The vernix caseosa, the waxy skin coating of a fetus, is shed into the amniotic fluid as the fetus approaches term. While still in utero, the near-term fetus swallows amniotic fluid containing pieces of vernix. The vernix is made up of short chain fatty acids (SCFAs), lipids which are unique to the vernix. While these vernix SCFAs are indigestible by human enzymes, they provide a rich medium for growth of bacteria15. When the infant is born, these SCFAs are already present in the gut, and act as the initial prebiotics for the infant. The importance of this role for the SCFAs is underscored in a recent study on a rat model of necrotizing enterocolitis (NEC), in which SCFAs were found to be protective. The protection was mediated by a change in intestinal microbial ecology, as well as an increase in regulatory cytokine interleukin 10 (IL-10)15.
Once the infant begins to breastfeed, breast milk contains additional prebiotics. Colostrum contains especially high concentrations of human milk oligosaccharides (HMOs), which are indigestible by human enzymes alone. Their synthesis requires up to ten percent of the total energy expended to produce human milk. These oligosaccharides, like the vernix SCFAs, promote growth of intestinal microbes16. In vitro, HMOs selectively promote growth of commensals such as Bifidobacterium longum subspecies infantis, while suppressing growth of pathogens like Escherichia coli and Clostridium perfringens17. The breakdown products of HMOs from B. infantis, lactate and SCFAs, also inhibit E. coli and C. perfringens growth. Furthermore, growth on HMOs rather than lactose alters the activity of B. infantis. B. infantis grown on HMOs bound more frequently to intestinal epithelial cells, and had stronger barrier-promoting and anti-inflammatory effects18.
Prebiotic compounds, such as SCFAs from the vernix caseosa and human milk oligosaccharides, give commensal bacteria an advantage over pathogens in the developing infant gut.
Breast milk
The myriad of benefits of breast milk in the early postnatal period have been covered in detail in two reviews16,19. We briefly summarize these benefits below and in Table 1.
Table 1.
Breast Milk Benefits
| Probiotic | Bacteria sampled from maternal gut directly inoculate infant gut |
| Prebiotic | Indigestible oligosaccharides |
| Innate immunity | Antimicrobial peptides, lactoferrin and lysozyme |
| Soluble CD14 recognizes LPS | |
| Soluble TLR2 | |
| Adaptive immunity | Secretory IgA specific to pathogens encountered in maternal gut |
| Glycan decoy cell adhesion molecules |
Maternal breast milk contains both innate and adaptive immune factors, which shape the infant microflora and protect against pathogenic infection. In addition, breast milk contains live bacteria translocated from the maternal gut and prebiotic oligosaccharides to encourage growth of commensals.
Breast milk has long been known to contain bacterial DNA. Modern culture-independent techniques identified gut-associated obligate anaerobes in addition to facultative anaerobes in the breast milk as a source of diversity in the infant gut20. Although viable bacteria are present in low concentrations20, maternal breast milk contains live, culturable bacteria including Bifidobacteria21. The bacteria were cultured from breast milk collected in a sterile manner, and were not found on the skin. It is believed that during pregnancy, increased intestinal permeability allows increased translocation of gut luminal bacteria to the lamina propria and Peyer’s patches22. These bacteria are believed to be engulfed by peripheral blood mononuclear cells (PBMCs)and transferred intact to the mammary gland, a process known as the enteromammary pathway. A study comparing pregnant women to non-pregnant women found bacterial DNA in both women’s PBMCs, but a greater abundance and diversity of bacterial DNA in those of pregnant women22,23. These studies in humans were complemented by studies of pregnant and lactating mice, which were found to have dramatically increased translocation of viable bacteria to the mesenteric lymph nodes and breast during pregnancy and lactation. The identification of shared species isolated from maternal fecal, peripheral blood and breast milk samples and infant fecal samples provide further evidence of this pathway as a source of bacterial inoculation for the infant gut.
During pregnancy, the T-helper (Th) cells of the maternal immune system are shifted away from Th1-type immunity and toward Th2-type immunity, in order to maintain tolerance of the developing fetus24. Th1 cells protect against intracellular pathogens and protozoa. Th2 cells interact with B cells to fight extracellular helminths. One might imagine that the skew of T helper cells away from Th1-type immunity during pregnancy could be responsible for maternal tolerance of viable bacteria within circulating macrophages. Th1 cytokine IL-12 was observed in a subset of the lactating mothers23, and caused speculation that in some cases, dysregulation of this permissive bacterial translocation could result in exaggerated Th1 cytokine production, followed by spontaneous abortion or preterm birth.
Through these prebiotic and probiotic mechanisms, breast milk shapes the intestinal microbiome. Exclusively breast-fed infants have overall greater diversity of the microbiome when compared to exclusively formula-fed infants25. Breast-fed infants also have a higher proportion of phylum Bacteroidetes, and a lower proportion of phylum Firmicutes. Microbiome research often focuses on the relative proportions between these two large groups, or phyla, of bacteria in causing disease. These microbial differences cause gene expression changes in the human host. While breast-fed infants paradoxically have a metagenome enriched in microbial virulence factors, these are in turn associated with a downregulation of host inflammatory genes25.
Breast milk contains both innate and adaptive immune components that inhibit pathogens from colonizing the infant gut. As part of the innate immune system, breast milk contains antibacterial peptides, such as lactoferrin and lysozyme. These antibacterial peptides provide broad spectrum bacteriostatic and bacteriocidal checks on microbial growth. Breast milk contains glycans that mimic cell surface adhesion molecules on the infant’s intestinal epithelium. These glycans act as decoy receptors to specific intestinal pathogens, providing a further level of protection for the infant26.
Breast milk also contains innate immune receptors, such as soluble CD14 (sCD14) and soluble Toll-like Receptor 2 (sTLR2)19. These soluble receptors act as part of a tightly-controlled immune response to colonizing microbes. sCD14 is a co-receptor which facilitates the response of TLR4 to lipopolysaccharide (LPS). sCD14 acts as a sentinel in the proximal gut lumen, and is necessary for inflammatory epithelial cytokine secretion (IL-8, TNF-α) in response to gram negative bacteria27. sCD14 is only partially resistant to degradation. It passes intact through the stomach, but is degraded by pancreatic enzymes before it reaches the bacteria-rich distal gut28. TLR4 is briefly upregulated in the early neonatal period, followed by downregulation19, which likely reflects the process of developing tolerance to commensals. sCD14 in the breast milk is similarly expressed at high levels in the first days after birth, after which it decreases gradually27.
A variety of innate immune receptors in the milk further modulate the immune response. sTLR2 interacts with sCD14 in breast milk to suppress the inflammatory response29. LPS binding protein (LBP), present at low concentrations in the breast milk, attenuates the sCD14-mediated TLR4 inflammatory response27. Another 80 kDa molecule present in breast milk, at present unidentified, increases TLR4 and TLR5 responsiveness, while decreasing TLR2 and TLR3 responses30. These receptors demonstrate the importance of breast milk in interacting with microbial colonization to effect immune homeostasis over the first days of the infant’s life.
In addition to the innate immune peptides and receptors, breast milk contains adaptive immune components. Specifically, maternal milk contains high levels of secretory IgA (sIgA). These sIgA are specific to those pathogens encountered by the mother’s own intestinal tract, targeting those pathogens the infant is most likely to encounter. Maternal gut exposure results in plasma cell production of sIgA in the mammary gland31. These sIgA pass intact through the distal gut28, where they affect microbial colonization in a lasting manner. In a mouse knockout model for the polymeric immunoglobulin receptor (Pigr), IgA was unable to be translocated from mammary gland into the breast milk32. The pups that did not receive passive transfer of sIgA in maternal milk had microbiota that were significantly different from controls. Furthermore, the microbial distance between groups magnified as the pups matured into adulthood, even as the pups began to produce endogenous IgA. Pups of Pigr knockout dams had decreased expression of epithelial maintenance genes, were more susceptible to intestinal invasion of aerobic bacteria, and more susceptible to chemically-induced colitis. These downstream effects underscore the importance of breast feeding on the microbiota, as well as the influence of the microbiota on gene expression and disease susceptibility.
Diet in later childhood
Much of what we know about the impact of diet on colonization comes from observing and comparing two populations. One study compared the microbiota of young European children from Italy with those of children from a rural African farming community in Burkina Faso. European children breast fed for a shorter time, ate more calories, more fat and animal protein, and less fiber when compared with those from Burkina Faso33. This resulted in a microbiome which was dramatically less diverse, with nearly opposite ratios of the bacterial phyla Bacteroidetes and Firmicutes (Italy: 22.4% Bacteroidetes, 63.7% Firmicutes; Burkina Faso: 57.7% Bacteroidetes, 27.3% Firmicutes). Microbiota with higher proportions of phylum Firmicutes, which includes Clostridia and Enterococcus, are associated with diseases including obesity34. Although these phylum-level differences are unlikely solely due to duration of breast feeding, this suggests a benefit to increased duration of breast feeding.
Another study of infants and adults from the United States, Amerindians from Venezuela and individuals from rural Malawi. The differences in gene expression in the microbiome reflect a striking adaptation of the microbiota to the different diets in these populations. Among infants, the presence of urease distinguished non-United States (Venezuelan and Malawian) infants from infants in the United States35. The presence of urease signifies nitrogen scavenging in a low-protein diet. Indeed, the United States has a comparatively high-protein diet compared to the Amerindian Venezuelan diet or rural Malawian diet. Similar differences were found among the microbiota of adults, with United States adults’ microbiota enriched in genes for amino acid degradation and simple sugar breakdown, whereas those from Venezuelan and Malawian adults had microbiota enriched in genes for amino acid synthesis and starch breakdown, again reflecting the diets of the different cultures35.
Normal colonization maintains homeostasis of the immune system
Microbiota stimulate the gastrointestinal immune system, which in turn protects against systemic immune responses to the gut microbiota
The microbiota play a role in shaping the architecture of the immune system (such as Peyer’s patches), the development of specific immune cell populations (such as regulatory T cells), and the balance between immune cell types. Two features of the gastrointestinal immune architecture affected by microbiota are the mucus layer and gut-associated lymphoid tissue (GALT), which includes Peyer’s patches.
Microbiota maintain a sterile mucus layer
The mucus layer maintains spatial segregation between the bacteria-rich gut lumen and the intestinal epithelium. This bacteria-free zone, approximately 50 micrometers thick, protects against otherwise continuous immune stimulation and inflammation. This serves to augment the barrier function of the epithelial layer, which is a single cell thick.
Intestinal microbes provide the stimuli for maintenance of the mucus layer. Germ-free animals have a thinner mucus layer than conventional animals36. Akkermansia muciniphila, which actually degrades mucin, is believed to increase the mucus layer’s thickness through increased turnover37. Similarly, probiotics have been shown to increase mucus production. In an intestinal epithelial cell line, lactobacillus increased mucin production38 by binding to the epithelial cells39. In doing so, these probiotics inhibited the adherence of enteropathogens enterohemorrhagic E. coli (EHEC) O157:H7 and enteropathogenic E. coli (EPEC)38.
The innate immune system maintains the sterility of the mucus layer. Intestinal epithelial cells (IECs)produce antibacterial RegIIIγ in a MyD88-dependent manner40. MyD88 is an adaptor protein in the TLR pathway. RegIIIγ is an antibacterial C-type lectin that targets gram-positive bacteria. MyD88 is both necessary and sufficient to main separation and prevent bacteria from interacting with epithelial cells. RegIIIγ knockout mice exhibited increased adaptive immune activation, as evidenced by increased fecal IgA and increased Th1 cells in the lamina propria; these increases were dependent on the presence of the intestinal microbiota. This physical segregation of the microbiota from the intestinal epithelium serves as an example by which the innate immune system maintains tolerance of the intestinal microbiota by limiting contact with the adaptive immune system.
Resistance to pathogens
A healthy microbiome further protects the host by forming its own protection against colonization. Commensals, like those in probiotics, can also protect against pathogens by preventing the pathogen from attaching to the colonic epithelium, a phenomenon known as colonization resistance41. As mentioned above, microbiota stimulate the mucus layer and also stimulate the epithelium to secrete antimicrobial peptides into this mucus layer, providing a barrier against pathogens38. Finally, commensals themselves can produce substances to block pathogenic infection. Commensal Bifidobacteria protect against enterohemorrhagic E. coli O157:H7 infection in a mouse model by production of acetate42.
Stimulation of GALT by microbiota
The microbiota stimulate the formation of GALT. As evidence of the role microbiota play in forming these immunologic structures, germ free animals have dramatically reduced germinal centers in the GALT, and reduced secretory IgA43,44.
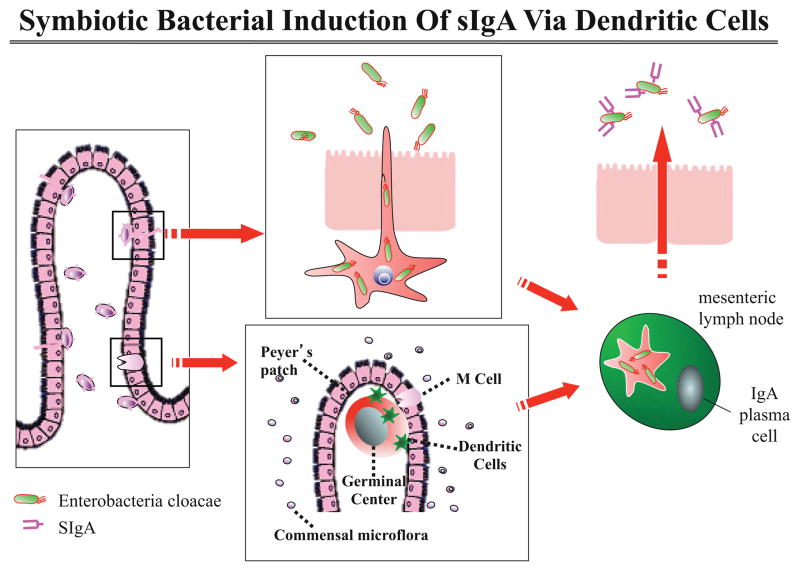
IgA produced by the GALT acts in an immunomodulatory manner45. Intestinal dendritic cells (DCs) sample the intestinal lumen. DCs that encounter bacterial polysaccharide A (PSA), a component of the capsular polysaccharide of commensal B. fragilis, stimulate the local adaptive immune system to produce secretory IgA. The locally-produced IgA coats the antigen, resulting in decreased activation of the innate immune response. This allows commensals to persist without detection by the systemic immune system.(Figure 1).
Figure 1. Symbiotic Bacterial Induction of sIgA Via Dendritic Cells.
Dendritic cells sample gut bacteria, which stimulates the formation of local sIgA by plasma cells. This sIgA is distributed along the length of the gut but not systemically. This allows the immune system to block antigenic stimulation at the epithelial surface and preserve systemic reactivity should the bacterium pass the epithelial barrier.
Adapted from Hooper et al. Science 2004;303:1662–65.
The GALT comprises a self-contained immune system, where antigens are recognized and generate an immune response, but the response (both the cells and the immunoglobulins they produce) is contained within the mucosal compartment46,47. The segregated circulation of cells within in the GALT concentrates the immune response in the gut, the site of the bacteria, at the same time avoiding a systemic inflammatory response.
These data find further support in epidemiologic studies. In a cohort of Swedish infants, increased diversity of Bifidobacterium species in early fecal samples is associated with increased salivary secretory IgA at 6 months of age48. This reflects the increased IgA production, distributed along the gastrointestinal tract. Increased salivary secretory IgA has been linked to protection against allergy49, as further evidence of local immunity protecting against systemic reactions.
Microbiota balance immune responses
Permissive colonization
In the perinatal period, the infant immune system shifts from one with a propensity to hyperstimulation50 to one of tolerance. The rapid colonization of the infant’s gut and other surfaces requires this shift. Elahi et al51 recently demonstrated one mechanism by which the neonatal gut allows colonization by microbes: a specific population of neonatal CD71+ erythroid cells dampen the innate immune response. This permissive microbial colonization (ideally by commensals) comes at the cost of an increased susceptibility to systemic infection in the neonatal period. The obvious magnitude of this cost underscores the evolutionary benefit of intestinal colonization in the neonatal period.
Anti-inflammatory stimuli
In addition to the immunomodulatory signals from the host during infancy, anti-inflammatory stimuli come from the microbiota as well. Short-chain fatty acids such as propionate and butyrate affect regulatory T cell (Treg) populations. High levels of SCFAs, produced when fermenting bacteria break down dietary fiber, also have immunomodulatory effects. Butyrate, in the presence of bacteria, increases the differentiation of progenitor cells to become Treg cells52. SCFAs specifically expanded the population of colonic Treg cells53. Both of these studies found an increase in IL-10 production with increased Treg cells52,53. SCFAs stimulated peripheral Treg generation (butyrate and propionate) and colonic accumulation (propionate and acetate) in microbiome-depleted mice54. Increased Treg differentiation was mediated by epigenetic changes to the FoxP3 gene locus54.
Finally, secreted products from Bifidobacteria have been shown to downregulate pro-inflammatory cytokines IL-8 and IFNγ, while upregulating anti-inflammatory cytokine IL-10 in a human fetal enterocyte line and fetal intestinal xenograft55. Early colonization with high levels of Bacteroides fragilis is associated with decreased expression of TLR4 mRNA, as well as with decreased cytokine responses to LPS48.
One of the persistent paradoxes in understanding the relationship between the host immune system and the microbiota remains how the host differentiates between commensals and pathogens. One such mechanism for differentiation involves recognition of bacterial DNA. Bacterial DNA from probiotics attenuates responses to inflammatory stimuli, whereas that from pathogens such as Salmonella augments the inflammatory response56. Pre-treatment of human intestinal epithelial cells with probiotic DNA before stimulation with proinflammatory TNF-α reduces IL-8 secretion, in a mechanism that involves decreased degradation of the inhibitory molecule IκB resulting in delayed activation of transcription factor NFκB. These observations were supported in a Crohn’s colitis model, the IL-10 knockout mouse. Oral administration of probiotic DNA to IL-10 knockout mice reduced TNF-α and IFN-γ mRNA as well as histologic injury scores56. This improvement in an in vivo Crohn’s colitis mouse model is encouraging for understanding therapeutic targets for IBD, especially given limited successes in treated IBD with probiotics57. These DNA-dependent effects are thought to be mediated by TLR9 recognition of unmethylated CpG motifs in DNA of pathogens56,58 yet more research is needed to identify the precise mechanism of this differentiation.
Microbes maintain balance between T helper cell subsets
The early gut microbiota also play a key role in regulating the balance between populations of CD4+ helper T-cells, Th1 and Th2 cells. As mentioned before, the maternal immune system is shifted toward Th2 type immunity during pregnancy24. This Th2-skewed cytokine milieu causes the infant immune system to also be shifted toward Th2-type immunity. During the first weeks and months of life, infants gradually increase Th1 activity, and restore balance of helper T cells. Without this shift, Th2-persistence is associated with atopic diseases including asthma59. A bacterially-derived carbohydrate is sufficient to cause this shift: Gut dendritic cells protruding through the intestinal epithelium sample commensal Bacteroides fragilis from the gut lumen. The sampled capsular polysaccharide A (PSA) from B. fragilis is transported to the systemic immune system, where it restores the balance between Th1 and Th2 cells in germ free mice60. Cesarean-born infants exhibit a delayed activation of Th1-type immunity, due to their altered colonization6.
Similarly, Th17 cells, inflammatory cells which protect against infection at mucosal surfaces, are also regulated by the microbiota. Specifically, Th17 cells are induced by segmented filamentous bacteria (SFB)61. In mice, SFB act via major histocompatibility complex class II (MHCII) on intestinal dendritic cells to increase differentiation of CD4+ T cells into Th17 cells in the lamina propria62. In addition, an unknown stimulus acts in an SFB-independent manner through MHCII located on innate lymphoid cells to inhibit Th17 differentiation; lineage-specific knockout of this MHCII also results in spontaneous Th17 activation62.
Appropriate colonization and immune response allows for optimal gut function
Through appropriate colonization and the resulting ‘education’ of the gastrointestinal immune system, infants develop more optimal gut function. These gut functions include vascular supply63, epithelial healing64 and nutrient absorption65.
The microbiota affect gene expression in host epithelial cells. In a landmark paper in 2001, germ free mice were monocolonized with B. thetaiotaomicron, which caused changes in gene expression of lipid metabolism66.
In another example, appropriate adaptive immune responses (IgA) to the gut microbiota are required to allow for lipid absorption and metabolism: Shulzhenko et al65 identified interconnected regulatory signaling networks, which balance innate defense mechanisms and metabolism in gut epithelial cells. In circumstances of decreased IgA, these regulatory networks become unbalanced. The lack of an adaptive immune response causes the gut epithelium to upregulate innate immune pathways, with a coincident downregulation of genes responsible for fat uptake and metabolism. This discovery helps to explain the lipid malabsorption and cachexia seen in patients with human immunodeficiency virus (HIV) and combined variable immunodeficiency (CVID). We hypothesize that the runaway immune activation seen in NEC and IBD could have similar effects on lipid absorption.
DYSBIOSIS AND ITS CONSEQUENCES: DISEASE
Dysbiosis is abnormal colonization, or the imbalance of microbes inhabiting a certain part of the body. The circumstances known to promote or to be associated with intestinal dysbiosis can be divided into the following categories based on their origin: 1)those which stem from abnormal microbial exposures; 2)disruptions in diet; 3)antibiotic usage and other medications; 4) the influence of host genetics. It is likely that there are other as-of-yet unknown causes as well.
Abnormal microbial exposures
Consequences of cesarean delivery
In 2010, a landmark study by Dominquez-Bello et al2 demonstrated that the intestinal microbiota of infants born by vaginal delivery closely resembled their mother’s vaginal microbiota, while those of infants born by cesarean instead reflected the microbes present in the infants’ environment (including Staph). Although the majority of mothers are presumed to have normal colonization, if the infant is born vaginally and the mother is in a state of dysbiosis, she will pass this on to her infant. In a mouse model, dysbiosis has been shown to transmit phenotypic traits such as allergy67 and obesity68 to the recipient mouse.
If the infant is born by cesarean section, the infant gut is colonized later by environmental rather than intestinal bacteria, and those bacteria are less diverse69,70. Infants born by cesarean section are specifically lacking in presence69 of and diversity70 within the Bacteroidetes phylum. While further research is needed to prove causality, cesarean birth has been associated with increased rates of later celiac disease71,72, obesity73 and asthma74, presumably through the microbiota. This association is particularly strong in cases of elective rather than emergent cesarean71; in a study of birth mode, microbial diversity was highest in emergent cesarean birth, followed by vaginal birth, with elective cesarean having the least diversity69. While many attribute this to the possibility of retrograde colonization with prolonged ruptured membranes in emergent cesarean, differences in hormones cannot be ruled out.
Decreased diversity
Twenty-five years ago David Strachan published the “Hygiene hypothesis,” now a familiar concept more accurately known as the “Microbial hypothesis.” He hypothesized that allergic diseases might be “prevented by infection in early childhood, transmitted by unhygienic contact with older siblings.”75 Further investigation has supported this concept, with new data providing support for an interaction between the high fat/high sugar Western diet, hygiene and the microbiota33,34,76.
Altered microbiota, such as that resulting from cesarean delivery, have been associated with numerous disease states. While the strength of these assertions varies, decreased diversity has been seen in diseases such as NEC77, colic78, inflammatory bowel disease (IBD)79, obesity1 and C. difficile colitis80.
There are several proposed mechanisms by which decreased diversity may cause disease. As described above, early intestinal bacteria act to modulate the immune system, resulting in maintenance of the sterile internal mucus layer40, increased secretory IgA48,45, colonization resistance41, immunomodulatory cytokines55, and decreased permeability81. Immune stimulation of the GALT actually results in decreased activation of the systemic immune system47. Insufficient diversity may weaken these beneficial immunomodulatory signals.
Delayed colonization
The timing of colonization matters because the immune system receives its microbial programming during the early neonatal period. Cesarean delivery results in delayed colonization, that is, a longer period after birth with fewer microbes and less diversity. There is a longer lag until the establishment of those intestinal anaerobes most commonly associated with the adult gut, such as Bacteroidetes6.
In mouse models, timing of colonization of germ free mice affects the ability of the microbiota to affect immune development. In one study, one week or more of germ free development (followed by conventionalization by cohousing) was sufficient to cause altered mononuclear cell populations and increased levels of regulatory cytokines82. One group of mice was inoculated with populations different from the animal facility’s typical microbiome. Those inoculated after three weeks showed persistently altered microbiota compared to the facility, whereas those inoculated at one week assimilated to the facility microbiome. Mice that remained germ-free for three weeks demonstrated greater inflammatory responses in cells harvested from the mesenteric lymph node, but also increased regulatory T cells and increased tolerogenic DCs82.
In a mouse model using oral antigen (OVA) exposure, intestinal colonization was required to induce systemic tolerance83. Mono-colonization of germ-free mice with Bifidobacterium infantis was sufficient to produce immune responsiveness, and therefore to induce tolerance to OVA. However, this effect was dependent upon colonization during infancy. When B. infantis was introduced in adulthood, the germ-free phenotype persisted, and oral OVA did not induce systemic tolerance.
In another study, germ-free (GF) mice were more susceptible to both ulcerative colitis and OVA-induced asthma than specific-pathogen free (SPF) mice84. This susceptibility was mediated by increased invariant natural killer T (iNKT) cells and increased chemokine ligand CXCL16. The susceptibility was diminished when the GF pups and their mothers were colonized on the day of birth, but not when the mice were colonized at five weeks of age. In further experiments, this immunologic programming was found to be at least partly mediated by time-sensitive epigenetic changes. Specifically, absence of colonization during the newborn period resulted in increased hypermethylation of the Cxcl16 gene, causing increased iNKT cell accumulation in the colon and lungs (Figure 2).
Figure 2. Early Colonization, Invariant Natural Killer T (iNKT) Cells in the Colon and Disease.
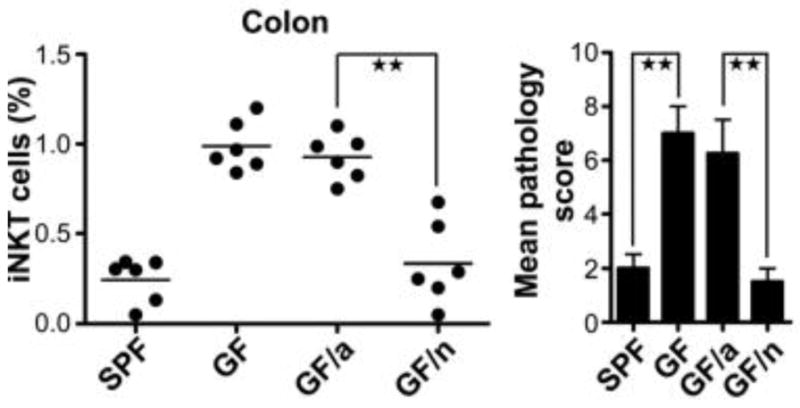
(Left) Colonization of germ free (GF) mice during the newborn period (GF/n) is sufficient to program the immune system to prevent iNKT cell accumulation in the colon. When GF mice are colonized as adults (GF/a), their immune system persists with the same phenotype as a GF mouse.
(Right) GF mice and GF/a mice are susceptible to ulcerative colitis. GF/n mice are protected from colitis by colonization in infancy.
Reproduced with permission from Olszak et al. Science 2012;336:489–493.
Taken together, these studies demonstrate the concept of a “window of opportunity” for microbial education of the developing immune system, which results in persistent alterations in systemic gene expression and, potentially, persistent changes in microbial populations. These data underscore the importance of the microbial health in the neonatal period. They also stimulate questions regarding probiotic therapy and microbial transplants. The circumstances of both donor and recipient could produce unforeseen effects on colonization, as well as on the resulting immunological changes in the recipient. For example, the ages and environmental circumstances (such as diet and microbial exposure) of both donor and recipient and the timing in relation to other microbial and immunological events (antibiotic therapy, pro- or anti-inflammatory milieu, immunosenesence) might all play a role in the success and safety of microbial transplants.
Nutritional contributions to dysbiosis
When to introduce antigens
The infant microbiome undergoes dramatic shifts upon introduction of solid foods7. This may represent a second window of development for the intestine and immune system, especially regarding oral tolerance85. The data on the timing of antigen introduction in order to reduce likelihood of an autoimmune or allergic reaction are not uniform86. Some circumstances suggest delaying antigen introduction: in a small randomized, controlled trial of infants at risk for celiac disease, the delayed introduction of gluten from 6 months to 12 months resulted in a decrease in the incidence of celiac disease at 2 years, as well as decreased development of anti-gliadin IgG antibodies87. The authors hypothesize that the genetic predisposition may have resulted in an immature, altered microbiome and provided a ripe environment for the development of gluten sensitization during infancy.
Conversely, recent observational studies suggest that earlier introduction of antigens may decrease food allergy. One study found that Israeli Jewish children, who have lower prevalence of peanut allergy, have significantly increased rates of early peanut consumption, whereas Jewish children in the UK, who have higher prevalence of peanut allergy, rarely consume peanuts during the first year of life88. Another study associated early introduction of complementary food (anything other than breast milk or formula before 4 months) with decreased rates of peanut-specific IgE in children with a parental history of allergy or asthma89. A third study of Finnish children at risk for type 1 diabetes found that earlier and more diverse introduction of complementary foods decreased risk of sensitization to several food and inhaled antigens90. These studies are in contrast to earlier recommendations of delaying introduction to potentially allergenic foods.
The role of the microbiome in the interaction between genetics and food antigens remains obscure and is a possible avenue for future investigation.
Nutrition and the mucus layer
Alterations in nutrition play an important role in moderating host-biome interactions. As described above, the intestinal mucus layer limits the stimulation of the host immune system by the microbiota. However, evidence is mounting that dietary emulsifiers can degrade the mucus layer. Emulsifiers serve to improve food texture, for example in ice cream. When added to mouse chow or water, emulsifiers decrease the mucus layer thickness and result in bacterial colonization of the inner mucus layer91. The changes in the mucus layer result in changes in the microbiota, as well as in effects of the microbiota on the host. Mice treated with emulsifiers have increased serum levels of flagellin and systemic inflammatory markers. While these mice do not exhibit histologic evidence of inflammation, mice treated with emulsifiers have shorter colons, which is a phenotypic marker of intestinal inflammation. Furthermore, these mice eat more and gain more weight than controls91.
Given this ground breaking evidence of the effects of emulsifiers on the mouse mucus layer, one can imagine the effects of dietary emulsifiers in humans. Excessive emulsifiers in processed foods may break down the barrier between intestinal biota and host defenses, resulting in increased immune stimulation, altered microbial populations and altered metabolism.
Thin mucus layers are found in sites of inflammation in adolescent patients with IBD when compared with non-inflamed sites and with controls with functional abdominal pain92. In patients with ulcerative colitis, sites of more severe inflammation have been observed to have thinner mucus layers93. When examining colonoscopic biopsies from adults94 and children95, patients with IBD were found to have significant bacterial colonization of the intestinal mucosal surface, whereas in controls the mucosa was nearly sterile. Furthermore, increasing mucosal bacteria were associated with increased severity of inflammation94. In an animal model of Crohn’s disease, a high fat/high sugar diet resulted in dysbiosis, decreased mucus layer thickness, increased permeability, and increased susceptibility to colonization with pathogenic E. coli96. Changes in the mucus layer may allow for increased systemic inflammation, with dramatic results on metabolism.
Nutrition, inflammation and obesity
A landmark paper associated chronic systemic inflammation with metabolic syndrome97. In a mouse model, levels of LPS in the blood (endotoxemia) were increased in mice fed a high fat diet, and this caused inflammation. The authors hypothesized that this endotoxemia was responsible for the metabolic syndrome seen in both humans and mice with high fat diets. The endotoxemia and systemic inflammation were recapitulated by direct subcutaneous infusion of LPS in mice. This resulted in increased fasting glucose, insulinemia, weight gain, adipose inflammation, hepatic insulin resistance and increased liver triglycerides – all markers of metabolic syndrome.
In a follow-up study, researchers determined that the microbiota of high fat diet mice provided the inflammatory stimulus for these metabolic changes98. Broad-spectrum antibiotics given to high fat diet mice reduced the intestinal bacterial load, which resulted in improved barrier function (less permeability), reduced endotoxemia and reduced inflammatory markers. Specifically, antibiotic treatment normalized levels of TNF-α, IL-1 and Plasminogen Activator Inhibitor-1 (PAI-1). PAI-1 is elevated in patients with metabolic syndrome and is thought to be at least partially responsible for increased cardiovascular disease seen in metabolic syndrome99. Antibiotic treatment also normalized markers of oxidative stress and macrophage infiltration in the visceral adipose tissue, with a subset of these also reduced in subcutaneous adipose tissue. These were associated with improved glucose tolerance, reduced insulin resistance and decreased visceral adiposity. Results in an ob/ob mouse model were similar to that of the high fat diet mouse model98.
A retrospective case-control study (sampled from a prospective study) compared the infantile microbiota of overweight/obese and normal weight children. Children were matched for mode of delivery, duration of breastfeeding, use of antibiotics and probiotics, and body mass index of the infant at birth. Children who became overweight/obese at 7 years of age had a trend towards lower proportions of Bifidobacteria and significantly higher concentrations of Staph aureus in their stool at 6 and 12 months of age100. As discussed later, Bifidobacteria are associated with improved barrier function and an improved metabolic profile101.
In obese patients (as well as in mice) there is a decrease in the ratio between the bacterial groups Bacteroidetes and Firmicutes (B:F ratio) as compared to lean patients who have a higher ratio34. Through dieting, obese patients gradually increased their B:F ratio. After a year of dieting and weight loss, the B:F ratio of obese patients was similar to that of lean patients.
This increased B:F ratio was also seen in African children from Burkina Faso when compared with European children from Italy33. Both the African children and obese patients on dietary therapy had increased fiber intake, decreased animal fats, and decreased total caloric intake, resulting in microbial change. As mentioned earlier, breast fed infants have an increased B:F ratio compared to non-breast fed infants25. This increased B:F ratio seen in infancy may initiate a cycle of microbiome affecting diet and metabolism, which in turn alters the microbiome, and help explain the protective effect of breastfeeding against developing obesity102,103.
Reinhardt et al104 outline how psychological stress affects obesity through the microbiome. Animal studies of newborn mouse pups and pregnant macaques demonstrate that stress results in dysbiosis. In the mouse studies, this dysbiosis resulted in increased intestinal permeability, akin to that seen in obesity. This dysbiosis and permeability was reversed by neonatal administration of probiotics.
Taken together, these nutritional studies demonstrate the critical importance of diet in influencing not only the mucus layer and intestinal health, but also metabolism. Childhood dietary choices interact with and have the potential to shift the microbiota established in infancy by breast-milk and environmental exposures, in turn shaping the child’s metabolic and immune profile105.
Antibiotic Usage
The effects of antibiotics on the intestinal microbiome provide further evidence for the importance of a diverse microbiome in maintaining homeostasis, particularly in the perinatal period. In a population-based cohort study of children in the United Kingdom (N=1,072,426), increased antibiotic use was associated with an increased risk of developing IBD106. The younger the age of exposure, the stronger the relationship was with development of IBD. The study identified anti-anaerobic antibiotic use as their primary analysis due to the observed specific lack of diversity in anaerobes seen in patients with active IBD79. The relationship persisted when examining any antibiotic use. Finally, adose-response relationship was observed: those with greater numbers of courses of anti-anaerobic antibiotics had increased hazard ratios of IBD. Of note, the study excluded antibiotics prescribed in the period immediately prior to diagnosis of IBD that may have been prescribed to treat symptoms of undiagnosed IBD. Without this exclusion, patients who received antibiotics leading up to their diagnosis of IBD may have falsely skewed the results toward an even greater association between antibiotics and IBD.
Similarly, a large, multi-center cohort analysis of extremely low birthweight infants (N=5693) found that prolonged empiric antibiotics increased the risks of NEC, death, and the composite outcome of NEC or death in a linear fashion107. For example, there was a ~7% increase in the odds of developing NEC for each additional day of empiric antibiotics. The association remained even after adjusting for sicker infants receiving longer courses of antibiotics.
Finally, a recent study in mice demonstrated that early antibiotic usage had lasting effects on immunity and metabolism due to transient changes in the microbiome108. Early exposure to low dose penicillin (LDP) contributed to obesity and decreased expression of several genes related to immunity. The timing of LDP exposure was significant, with earlier penicillin exposure resulting in greater metabolic changes. The effects of LDP were additive with diet, such that LDP-exposed mice fed a high fat diet had greater fat mass accumulation than those either fed a high fat diet or exposed to LDP alone. The metabolic changes were due to alterations in the microbiota: when the microbiota from LDP-exposed mice were transplanted into germ free mice, the metabolic and immune changes were recapitulated. Perhaps most importantly, short periods of LDP exposure resulted in “durable phenotypes.” Despite normalization of the microbiota after cessation of antibiotics, the phenotypes of elevated fat mass and decreased expression of immune-related genes persisted. This study incorporates several important concepts related to infant colonization including synergy of microbiota and diet, transmissibility of phenotypes through the microbiome and most importantly the window of opportunity for regulation of the immune system and metabolism.
Genetics and dysbiosis
While research into the effects of dysbiosis on the host abounds, the effects of the host on the microbiome are more limited. In a mouse model of genetic susceptibility to food allergy, the baseline microbiome of genetically susceptible mice differed significantly compared to wild type (WT) mice67. Upon sensitization with allergen ovalbumin (OVA), the microbiome of allergy-prone mice shifted further from baseline.
Dysbiosis resulting from genetic mutations have also been shown to be transmissible. Genetically obese mice had microbiota with an increased capacity for energy harvest compared to lean littermate mice and were not attributable to differences in food consumption in this study68. This increased capacity for energy harvest was demonstrated by the decreased energy remaining in cecal contents of obese mice. That this microbial population was responsible for the obese phenotype was confirmed by microbial transplant. Germ free mice that received microbiota from obese mice increased their body fat by 47%, significantly greater than those which received lean microbiota (27% increase). There was no difference in diet between the two groups.
Similar studies in mouse models of Crohn’s disease109 and ulcerative colitis110 provide further evidence of the effects of the host on the microbiome. Each study demonstrates that genetic mutations cause dysbiosis, which can then be transplanted into GF wild-type mice, and recapitulate the phenotype seen in mice with that genetic mutation.
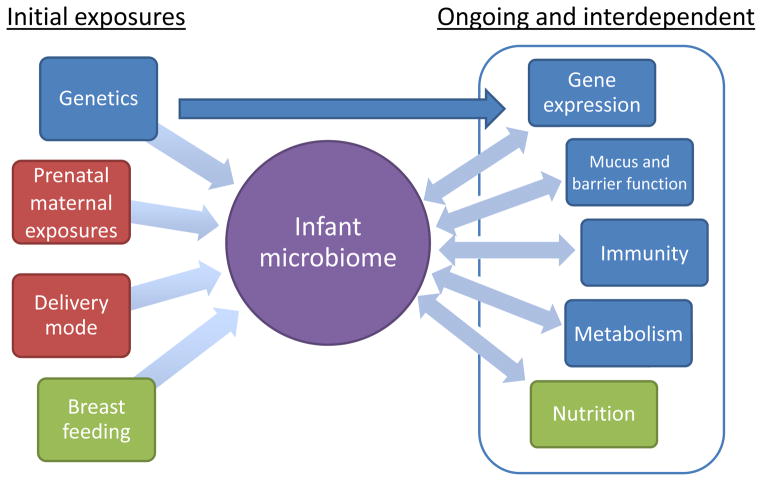
These genetic studies contribute to the concept recently described by Hooper46, that the host exerts inside-out control over the microbiota, while the microbiota also exert outside-in programming of host immunity and metabolism. This cycle of cross-talk between the microbiota, host genetics, nutrition, immunity and metabolism is initiated in infancy (Figure 3). The window of opportunity to establish host immunity, and therefore inside-out control of the microbiome, whose effects will persist throughout life, depends on appropriate infant colonization through prenatal maternal exposures9, delivery mode2, breast feeding14,16,19 and judicious use of antibiotics106.
Figure 3. Cross-talk between microbiome and intestinal homeostasis.
Initial infant colonization results from genetics, microbial exposures such as delivery mode and antibiotic usage, and breast feeding. This, in turn, sets in motion the cross-talk between the microbiome, nutrition, immunity, barrier function, metabolism and gene expression. The initial colonization of the infant and microbiome-directed therapies represent a major avenue for prevention and treatment of immune and metabolic disease.
POTENTIAL SOLUTIONS AND RECOMMENDATIONS
Treatments for dysbiosis are in their infancy. Ideally, in the future, treatments will be tailored to the cause of dysbiosis and will reflect knowledge of microbial-gut homeostasis. Patients are aware of microbial therapies such as probiotics and fecal microbiota transplant111. Physicians have a responsibility to inform themselves about these therapies and, in turn, educate their patients as to the risks and benefits of microbial therapy.
When dysbiosis has already occurred, there are two major categories of treatment: nutritional changes to encourage the growth of normal endogenous microbes and direct administration of live microorganisms.
Nutritional measures that directly affect the microbiome
Breast feeding remains one of the most important factors in determining initial infant colonization and future health23. In cases where breast milk is not available, donor human breast milk represents a preferred alternative to infant formula, especially in cases of prematurity112. However, donor breast milk and maternal breast milk exhibit important differences. The immunologic composition of breast milk changes significantly depending on time since delivery and gestational age at delivery113. For example, levels of sIgA, IgG, and many growth factors are all increased in breast milk from mothers of preterm infants, and are highest in colostrum, tapering with increasing time since delivery. Conversely, lactoferrin is decreased in milk from mothers who delivery prematurely compared to that from mothers who deliver at term, and also tapers over time. Cytokine profiles also vary with gestational age and time since delivery. In addition, the bacterial inoculum in mother’s milk, which includes bacteria sampled from the maternal gut, is killed during donor milk pasteurization114. Given the differences in adaptive and innate immune components of milk depending on gestational age at delivery and time since birth, compounded by pasteurization, donor milk remains an imperfect substitute for maternal breast milk, particularly in the preterm infant113.
The effects of diet as an independent driver of the microbiome are well established. In a simplified mouse model of microbial populations, changes in diet predicted more than half of observed changes in the microbiome115. Mice were colonized with ten species common to the healthy human gut, with varying metabolic functions. Mice were fed combinations of four purified macronutrients: casein, corn oil, cornstarch and sucrose. The observed changes were sufficient data to produce a linear model of microbial population change, which was then used to predict changes in the mouse microbiome in response to different macronutrient components of the diet. The experiment was repeated using common pureed baby foods in place of purified macronutrients115.
Levels of fiber, fat and animal protein are often cited as the major differences and drivers of microbial change between human diets. Low levels of fiber in the Western Diet are thought to decrease substrates for the production of SCFAs, and, therefore lead to decreased SCFAs and decreased Treg cells53. In another example showing the impact of diet, in a study of elderly people, those who lived in the community had much more diverse microbiota than those who lived in long-term care facilities76. Upon closer examination, the location of dwelling was not the primary driver of microbial change. Rather, the change in diet from one of high fiber with low or moderate fat to one of low fiber with moderate or high fat resulted in a dramatic shift away from the community-associated microbiome to one associated with long-term care facilities.
Initial observations of children undergoing nutritional therapy for Crohn’s disease suggest that changes to the microbiome are associated with clinical improvement. Nutritional therapy results in significant changes in the microbiome116, with increased Bacteroidetes and increased overall diversity117. There is currently a larger study to examine the effects of elemental diet therapy for pediatric IBD on the microbiome118.
Prebiotics
Prebiotics alter the ratios of endogenous bacteria in the intestine, in turn affecting gut function and metabolism. As described in the beginning of this review, prebiotics in the vernix caseosa and breast milk promote growth of commensals such as B. longum17. Prebiotics can correct bacterial imbalances with far-reaching effects. For example, the prebiotic oligofructose (OFS) increases the proportion of colonic Bifidobacteria, improves gut barrier function, decreases endotoxemia, and improves glucose tolerance101,104,119. These benefits of OFS and Bifidobacteria are mediated through glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). GLP-1 and GLP-2 are gastrointestinal hormones that decrease serum glucose and promote satiety. The effects on glucose and satiety in turn alter host metabolism and diet. One might speculate that the anti-inflammatory properties of the secreted products of Bifidobacteria seen in the immature gut55 also play a role in mediating the benefits of prebiotics.
Prebiotics have been shown to increase absorption of minerals, especially calcium120. Prebiotics, in combination with probiotics (so-called synbiotics) have also demonstrated efficacy in treatment of ulcerative colitis121. Prebiotics generally have mild adverse effects, such as flatulence and increased stool output when taken in larger amounts120. Despite this, and given the unknown risks associated with administration of microbial communities, prebiotics represent an appealing and safe therapeutic option for treatment of dysbiosis.
Microbial therapies
Maternal microbiota
The state of the maternal microbiota affects the colonization of the infant from birth. As such, the prenatal and even pre-pregnancy maternal microbiota represents a target in the prevention of infant dysbiosis. As mentioned before, maternal farming exposures and consumption of raw milk modulate infant immunity as demonstrated by decreased risk of atopic disease9. However, further research is needed before widespread adoption of maternal interventions such as probiotic use.
A study is currently ongoing to evaluate the exogenous administration of vaginal secretions to infants delivered by cesarean section122. This study could lead to an exciting change in practice. However, in certain circumstances, the cause of cesarean delivery, such as macrosomia or preterm delivery, could be due to maternal dysbiosis, such as diabetes98 or infection of the birth canal123. Further research will be needed before changes can be implemented widely.
Probiotics
In theory, probiotics represent a tempting fix to complex dysbiosis: identify the missing bacteria and replace them. In practice, this has proved more difficult. Probiotics have been found to be effective in some diseases, such as irritable bowel syndrome and pouchitis. However, results in other diseases such as Crohn’s have been disappointing41, likely due to the variability in pathogenesis and in the course of Crohn’s disease. The treatment of NEC with probiotics or probiotic-secreted factors remains a tantalizing possibility55. The mechanisms of action of probiotics are diverse and include nearly every effect on the host as by the endogenous microbiome. These include stimulation of mucus production38, inhibition of pathogen adherence39 and improved barrier function18. Probiotics downregulate expression of innate immune receptors41, balance subsets of T helper cells60, and alter gene expression66.
Despite more than a decade of basic research and clinical trialson a range of single-organism and cocktail probiotics41, the efficacy of a probiotic for a given condition remains hard to predict. The adult microbiome is stable and relatively difficult to perturb8. Evidence for treatment of conditions such as Crohn’s disease are equivocal57. In one notable trial, probiotics in the setting of severe pancreatitis has been shown to be harmful41. Given these mixed results, probiotic research remains in a guess-and-check stage. However, those probiotics which have been shown to be helpful, such as combination probiotic VSL#3 for pouchitis, or Lactobacillus GG and B. lactis for allergy, may be used with confidence124.
Helminths
The use of helminths as an anti-inflammatory therapy has caught the attention of both physicians and the public. Research into the anti-inflammatory properties of helminth infection is inspired by the hygiene hypothesis75, and the eradication of helminths in the industrialized world, where autoimmune diseases are most prevalent. Indeed, a recent case control study in South Africa found childhood helminth infection to be protective against IBD (adjusted odds ratio = 0.2)125. The majority of studies use the porcine whipworm, Trichuris suis, due to its accessibility in large numbers, its ability to temporarily and safely colonize humans, and the lack of transmission between close human contacts126. However, current data are extremely limited. A randomized, controlled trial of 54 patients with ulcerative colitis demonstrated improvement in 13/30 (43.3%) patients who received T. suis ova (TSO), versus improvement in 4/24 (16.7%) patients in the placebo arm127. An open-label trial of TSO administration in 29 patients with Crohn’s disease showed a 79.3% response rate (23/29 patients)128. These and further studies129 have found T. suis therapy to be safe, even with patients concurrently taking immunosuppressants.
The mechanisms of helminth therapy are just beginning to be elucidated. Another worm, Heligmosomoides polygyrus bakeri, was found to induce activation of Foxp3+ regulatory T cells in a mouse model130. These Treg cells were transplanted into Rag mice, where they prevented piroxicam-induced colitis. Treg cells from uninfected mice were not protective.
Further studies suggest that administration of secreted/excreted worm products may be sufficient to produce anti-inflammatory effects. Secreted/excreted products from T. suis were applied to mouse intestinal epithelial cells. This reduced the inflammatory reaction to LPS, although surprisingly it increased the permeability of the epithelial layer131.
Despite these early promising studies, study cohorts for helminth therapy have so far been small, and study durations short. There is limited understanding of mechanisms of action of helminths therapies. Furthermore, nearly all of these investigations have originated from the same group of scientists. Further studies are needed at multiple sites, with more patients and longer trials.
Fecal Microbiota Transplant
Fecal microbiota transplant (FMT) has demonstrated efficacy in adults for recurrent C. difficile colitis132. Public interest in FMT has grown111 and FMT is proposed for an increasingly diverse range of pediatric conditions133. However, evidence in pediatric patients is limited to a few cases. Given the interest in FMT coupled with the limited data in pediatric FMT, we will cover this topic in detail.
In 2010, Russell et al134 reported a case of a two year old female with relapsing C. difficile infection, refractory to 8 months of traditional and experimental antibiotics and probiotics. After appropriate donor screening, a single FMT from the patient’s father via nasogastric tube was effective.
An open-label pilot study of ten children, age 7–21, evaluated FMT in mild-to-moderate ulcerative colitis (UC)135. Five consecutive daily treatments were administered by enema from screened donors. One patient could not retain the enema and was excluded. 7 of 9 patients responded to FMT at 1 week, of which 6 maintained the response at 1 month. 3 of 9 patients reported remission at 1 week. Adverse effects included fever in two patients, which were controlled with antipyretics.
Most recently, a one-year old with early-onset colitis was treated with FMT136. The patient presented with bloody diarrhea at 10 months, refractory to mesalamine, azathioprine, prednisolone, infliximab, probiotics, antibiotics and amino acid based formula. Genetic conditions and infectious causes were ruled out. At age 18 months, the patient received four courses of FMT from an age-matched cousin with temporary response. The first course resulted in a marked reaction with profuse sweating, vomiting, paleness, tachycardia and fever. This reaction recurred but diminished with the following two treatments. After a total of 7 treatments the patient was able to taper off medications. The patient was asymptomatic with normal histology 6 months after the last FMT, 3 months after stopping all medications.
These limited data demonstrate the variable response to FMT, as well as a potential for inflammatory reactions to treatment. Although no major adverse events such as sepsis have been reported, the UC study was careful to exclude patients on anti-TNF therapy or patients with severe disease135. All studies performed careful screening of donors134.
The mechanism of improvement in FMT is still unknown. Longer-term efficacy and safety profiles of FMT are also unknown. As demonstrated in this review, dysbiosis can occur secondary to altered immunity, diet, or genetics, with potentially insidious effects on host metabolism and immunity. Related microbial donors may share a genetic mutation with the patient, resulting in a similar dysbiosis. The microbiome has been implicated in diseases with insidious onset, such as cardiovascular disease137, making adverse effects of FMT difficult to track. Finally, it is currently unknown which bacteria may be commensals in one patient, but pathogens in another.
Given the enthusiasm for and perception of FMT as “natural” demonstrated by patients111, we recommend open discussion of FMT with patients, but reservation of FMT for cases where traditional treatments have been proven futile.
Conclusions
The mechanisms of relationships between host, microbiota and environment are gradually being revealed. Infant exposures set into motion simultaneous and reciprocal development of the immune system, gastrointestinal tract and metabolism. The exposures affecting the infant microbiota, including maternal exposures, birth mode, breast feeding, and antibiotics continue to affect the individual and his or her physiology throughout childhood and into adulthood. Treatment options for dysbiosis have gained greater awareness and acceptance in the general population. However, evidence from human trials of therapeutic helminths and FMT are based on small numbers of patients. These therapies should be exercised with caution in patients for whom traditional therapies have been exhausted.
Acknowledgments
Supported by NIH grants (P30 DK040561; P01 DK033506; R01 HD012437; R01 HD059126; 3T32DK007191-39S1)
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest.
References
- 1.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CL, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. 2012;129(5):950–60. doi: 10.1542/peds.2011-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jost T, Lacroix C, Braegger CP, et al. New Insights in Gut Microbiota Establishment in Healthy Breast Fed Neonates. PLoS One. 2012;7(8):e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035s–45. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut. 2013;0:1–8. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 7.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faith JJ, Guruge JL, Charbonneau M, et al. The Long-Term Stability of the Human Gut Microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Mutius E. Maternal farm exposure/ingestion of unpasteurized cow’s milk and allergic disease. Curr Opin Gastroenterol. 2012;28:570–6. doi: 10.1097/MOG.0b013e32835955d3. [DOI] [PubMed] [Google Scholar]
- 10.Funkhouser LJ, Bordenstein SR. Mom Knows Best: The Universality of Maternal Microbial Transmission. PLoS Biology. 2013;11(8):e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardissone AN, de la Cruz DM, Davis-Richardson A, et al. Meconium Microbiome Analysis Identifies Bacteria Correlated with Preterm irth. PLoS One. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rautava S, Luoto R, Salminen S, et al. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–576. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 13.Makino H, Kushiro A, Ishikawa E, et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS One. 2013;8(11):e78331. doi: 10.1371/journal.pone.0078331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez PF, Doré J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119(3):e724–32. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 15.Ran-Ressler RR, Khailova L, Arganbright KM, et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PloS ONE. 2011;6(12):e29032. doi: 10.1371/journal.pone.0029032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z, Chen C, Kling DE, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiol. 2013;23(2):169–77. doi: 10.1093/glycob/cws138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chichlowski M, De Lartigue G, German JB, et al. Bifidobacteria Isolated From Infants and Cultured on Human Milk Oligosaccharides Affect Intestinal Epithelial Function. J Pediatr Gastroenterol Nutr. 2012;55(3):321–7. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnet-Hughes A, Schiffrin EJ, Walker WA. Protective Properties of Human Milk and Bacterial Colonization of the Neonatal Gut. In: Duggan C, Watkins J, Walker WA, editors. Nutrition in Pediatrics. 5. Shelton: BC Decker; 2014. In press. [Google Scholar]
- 20.Jost T, Lacroix C, Braegger C, et al. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Brit J Nutr. 2013;110:1253–1262. doi: 10.1017/S0007114513000597. [DOI] [PubMed] [Google Scholar]
- 21.Martín R, Jiménez E, Heilig H, et al. Isolation of Bifidobacteria from Breast Milk and Assessment of the Bifidobacterial Population by PCR-Denaturing Gradient Gel Electrophoresis and Quantitative Real-Time PCR. Appl and Enviro Microbiol. 2009;75(4):965–9. doi: 10.1128/AEM.02063-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez PF, Doré J, Leclerc M, et al. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics. 2007;119(3):e724–32. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 23.Donnet-Hughes A, Perez PF, Doré J, et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nut Soc. 2010;69:407–415. doi: 10.1017/S0029665110001898. [DOI] [PubMed] [Google Scholar]
- 24.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz S, Friedberg I, Ivanov IV, et al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13(4):r32. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow AL, Ruiz-Palacios GM, Altaye M, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145(3):297–303. doi: 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Vidal K, Labéta MO, Schiffrin EJ, et al. Soluble CD14 in human breast milk and its role in innate immune responses. Acta Odontol Scand. 2001;59:330–4. doi: 10.1080/000163501750541219. [DOI] [PubMed] [Google Scholar]
- 28.Blais DR, Harrold J, Altossar I. Killing the Messenger in the Nick of Time: Persistence of Breast Milk sCD14 in the Neonatal Gastrointestinal Tract. Pediatr Res. 2006;59(3):371–6. doi: 10.1203/01.pdr.0000199907.61549.94. [DOI] [PubMed] [Google Scholar]
- 29.LeBouder E, Rey-Nores JE, Rushmere NK. Soluble Forms of Toll-Like Receptor (TLR) 2 Capable of Modulating TLR2 Signaling Are Present in Human Plasma and Breast Milk. J Immunol. 2003;171:6680–9. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 30.LeBouder E, Rey-Nores JE, Raby A, et al. Modulation of Neonatal Microbial Recognition: TLR-Mediated Innate Immune Responses Are Specifically and Differentially Modulated by Human Milk. J Immunol. 2006;176:3742–52. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 31.Hanson LÅ. Comparative immunological studies of the immune globulins of human milk and of blood serum. Int Arch All Immunol. 1961;18(5):241–267. doi: 10.1159/000229177. [DOI] [PubMed] [Google Scholar]
- 32.Rogier EW, Frantz AL, Bruno MEC, et al. Secretory antibodies in breast milk promote long term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci. 2014;111(8):3074–9. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Nat Acad Sci. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 35.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–41. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 37.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack DR, Michail S, Wei S, et al. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol Gastrointest Liver Physiol. 1999;276(4):G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 39.Mack DR, Ahrné S, Hyde L, et al. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–33. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaishnava S, Yamamoto M, Severson KM, et al. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7:503–14. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–9. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 43.Cebra JJ, Periwal SB, Lee G, et al. Development and Maintenance of the Gut- Associated Lymphoid Tissue (GALT): The Roles of Enteric Bacteria and Viruses. Dev Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibnou-Zekri N, Blum S, Schiffrin EJ, et al. Divergent Patterns of Colonization and Immune Response Elicited from Two Intestinal Lactobacillus Strains That Display Similar Properties In Vitro. Infect Immun. 2003;71(1):428–36. doi: 10.1128/IAI.71.1.428-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson DA, McNulty NP, Guruge JL, et al. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–39. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacPherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann N Y Acad Sci. 2004;1029(1):36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 48.Sjögren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp All. 2009;39(12):1842–51. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 49.Böttcher MF, Häggström P, Björksten B, et al. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp All. 2002;32(9):1293–1298. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 50.Nanthakumar N, Meng D, Goldstein AM, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PloS ONE. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504(7478):158–62. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 53.Smith PM, Howitt MR, Panikov N, et al. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–55. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ganguli K, Meng D, Rautava S, et al. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;304(2):G132–41. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jijon H, Backer J, Diaz H, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126(5):1358–73. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Isaacs K, Herfarth H. Role of Probiotic Therapy in IBD. Inflamm Bowel Dis. 2008;14:1597–1605. doi: 10.1002/ibd.20465. [DOI] [PubMed] [Google Scholar]
- 58.Kant R, de Vos WM, Palva A, et al. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J Med Microbiol. 2014;63(Pt 2):293–308. doi: 10.1099/jmm.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 59.Robinson DS, Hamid Q, Ying S, et al. TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 60.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Ivanov II, Atarashi K, Manel N, et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goto Y, Panea C, Nakato G, et al. Segmented Filamentous Bacteria Antigens Presented by Intestinal Dendritic Cells Drive Mucosal Th17 Cell Differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci. 2002;99(24):15451–5. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pull SL, Doherty JM, Mills JC, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci. 2005;102(1):99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shulzhenko N, Morgun A, Hsiao W, et al. Cross talk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat med. 2011;17(12):1585–93. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291(5505):881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 67.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J All Clin Immunol. 2013;131(1):201–12. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 69.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut. 2013;63(4):559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 71.Mårild K, Stephansson O, Montgomery S, et al. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142(1):39–45. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Decker E, Engelmann G, Findeisen A, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125(6):e1433–40. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 73.Mesquita DN, Barbieri MA, Goldani HA, et al. Cesarean section is associated with increased peripheral and central adiposity in young adulthood: cohort study. PloS ONE. 2013;8(6):e66827. doi: 10.1371/journal.pone.0066827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thavagnanam S, Fleming J, Bromley A, et al. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 75.Strachan DP. Hay fever, hygiene and household size. Br Med J. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Science. 2012;488:178–84. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The ISME Journal. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Weerth C, Fuentes S, Puylaert P, et al. Intestinal Microbiota of Infants with Colic: Development and Specific Signatures. Pediatrics. 2013;131(2):e550–8. doi: 10.1542/peds.2012-1449. [DOI] [PubMed] [Google Scholar]
- 79.Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–93. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 81.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2 driven improvement in gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen CHF, Nielsen DS, Kverka M, et al. Patterns of Early Gut Colonization Shape Future Immune Responses of the Host. PLoS ONE. 2012;7(3):e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sudo N, Sawamura S, Tanaka K, et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159(4):1739–1745. [PubMed] [Google Scholar]
- 84.Olszak T, An D, Zeissig S, et al. Microbial Exposure During Early Life Has Persistent Effects on Natural Killer T Cell Function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Penders J, Stobberingh EE, van den Brandt, et al. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 86.Szajewska H. The prevention of food allergy in children. Curr Opin Clin Nutr Metab Care. 2013;16(3):346–50. doi: 10.1097/MCO.0b013e32835e365f. [DOI] [PubMed] [Google Scholar]
- 87.Sellitto M, Bai G, Serena G, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PloS ONE. 2012;7(3):e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du Toit G, Katz Y, Sasieni P, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 89.Joseph CLM, Ownby DR, Havstad SL, et al. Early complementary feeding and risk of food sensitization in a birth cohort. J Allergy Clin Immunol. 2011;127(5):1203–10. doi: 10.1016/j.jaci.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nwaru BI, Takkinen HM, Niemelä O, et al. Introduction of complementary foods in infancy and atopic sensitization at the age of 5 years: timing and food diversity in a Finnish birth cohort. Allergy. 2013;68:507–16. doi: 10.1111/all.12118. [DOI] [PubMed] [Google Scholar]
- 91.Gewirtz A. Microbiota, Low-Grade Inflammation, and Metabolic Syndrome. Lecture conducted from Harvard Medical School; The 14th Annual Postgraduate Nutrition Symposium: Metabolic Syndrome; Boston, MA. 2013. Jul, http://nutrition.med.harvard.edu/education/edu_nut_symp14_program.html. [Google Scholar]
- 92.Fyderek K, Strus M, Kowalska-Duplaga K, et al. Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J of Gastroenterol. 2009;15(42):5287–5294. doi: 10.3748/wjg.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pullan RD, Thomas GA, Rhodes M, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122(1):44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 95.Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55(12):1760–1767. doi: 10.1136/gut.2005.078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martinez-Medina M, Denizot J, Dreux N, et al. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonization. Gut. 2014;63:116–24. doi: 10.1136/gutjnl-2012-304119. [DOI] [PubMed] [Google Scholar]
- 97.Cani PD, Amar J, Angel Iglesias M, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 98.Cani PD, Bibiloni R, Knauf C, et al. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 99.Juhanvague I, Thompson SC, Jespersen J. Involvement of the Hemostatic System in the Insulin-resistance Syndrome – A Study of 1500 Patients with Angina-Pectoris. Arterioscler Thromb. 1993;13(12):1865–73. doi: 10.1161/01.atv.13.12.1865. [DOI] [PubMed] [Google Scholar]
- 100.Kalliomäki M, Collado MC, Salminen S, et al. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 101.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owen CG, Martin RM, Whincup PH, et al. Effects of Infant Feeding on the Risk of Obesity Across the Life Course: A Quantitative Review of Published Evidence. Pediatrics. 2005;115:1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 103.Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9(2):135–46. doi: 10.1111/j.2047-6310.2013.00155.x. [DOI] [PubMed] [Google Scholar]
- 104.Reinhardt C, Reigstad CS, Bäckhed F. Intestinal Microbiota During Infancy and Its Implications for Obesity. J Pediatr Gastroenterol Nutr. 2009;48(3):249–56. doi: 10.1097/mpg.0b013e318183187c. [DOI] [PubMed] [Google Scholar]
- 105.Shanahan F. The gut microbiota—a clinical perspective on lessons learned. Nat Rev Gastroenterol Hepatol. 2012;9(10):609–14. doi: 10.1038/nrgastro.2012.145. [DOI] [PubMed] [Google Scholar]
- 106.Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–e803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cox LM, Yamanishi S, Sohn J, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Couturier-Maillard A, Secher T, Rehman A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123(2):700–11. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae Act in Concert with the Gut Microbiota to Induce Spontaneous and Maternally Transmitted Colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis. 2012;18(4):676–84. doi: 10.1002/ibd.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F169–75. doi: 10.1136/adc.2005.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gregory KE, Walker WA. Immunologic Factors in Human Milk and Disease Prevention in the Preterm Infant. Curr Pediatr Rep. 2013;1(4):222–8. doi: 10.1007/s40124-013-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landers S, Updegrove K. Bacteriological Screening of Donor Human Milk Before and After Holder Pasteurization. Breastfeed Med. 2010;5(3):117–21. doi: 10.1089/bfm.2009.0032. [DOI] [PubMed] [Google Scholar]
- 115.Faith JJ, McNulty NP, Rey FE, et al. Predicting a Human Gut Microbiota’s Response to Diet in Gnotobiotic Mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lionetti P, Callegari M, Cavicchi M, et al. Enteral Nutrition-Induced Remission Is Associated with Profound Modification of Intestinal Microflora in Crohn’s Disease. J Pediatr Gastroenterol Nutr. 2004;39:S106. [Google Scholar]
- 117.D’Argenio V, Precone V, Casaburi G, et al. An Altered Gut Microbiome Profile in a Child Affected by Crohn’s Disease Normalized After Nutritional Therapy. Am J Gastro. 2013;108(5):851–2. doi: 10.1038/ajg.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu GD, Lewis JD, Bushman FD. Diet, Genetic Factors, and the Gut Microbiome in Crohn’s Disease. [Accessed April 18, 2014];National Center for Biotechnology Information Web site. http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000252.v2.p1.
- 119.Cani PD, Kanuf C, Iglesias MA, et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes. 2006;55:1484–90. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 120.Teitelbaum JE, Walker WA. Nutritional Impact of Pre- and Probiotics as Protective Gastrointestinal Organisms. Ann Rev Nutr. 2002;22:107–38. doi: 10.1146/annurev.nutr.22.110901.145412. [DOI] [PubMed] [Google Scholar]
- 121.Furrie E, Macfarlane S, Kennedy A, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54(2):242–49. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dominguez-Bello M. Human Microbiome Science: Vision for the Future. Lecture conducted from University of Maryland School of Medicine. Bethesda, MD: 2013. Jul, The Modern vs. Ancestral Microbiome. [Google Scholar]
- 123.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–07. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 124.Floch MH, Walker WA, Madsen K, et al. Recommendations for Probiotic Use—2011 Update. J Clin Gastroenterol. 2011;45:S168–71. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 125.Chu KM, Watermeyer G, Shelly L. Childhood Helminth Exposure Is Protective Against Inflammatory Bowel Disease: A Case Control Study in South Africa. Inflamm Bowel Dis. 2013;19:614–20. doi: 10.1097/MIB.0b013e31827f27f4. [DOI] [PubMed] [Google Scholar]
- 126.Elliott DE, Summers RW, Weinstock JV. Helminths as Governors of Immune-mediated Inflammation. Int J Parasitol. 2007;37:457–64. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 127.Summers RW, Elliott DE, Urban JF, et al. Tricuris suis Therapy for Active Ulcerative Colitis: A Randomized Controlled Trial. Gastroenterol. 2005;128(4):825–32. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Summers RW, Elliott DE, Urban JF, et al. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sandborn WJ, Elliott DE, Weinstock J, et al. Randomised clinical trial: the safety and tolerability of Tricuris suis ova in patients with Crohn’s disease. Aliment Pharm Therapeu. 2013;38:255–263. doi: 10.1111/apt.12366. [DOI] [PubMed] [Google Scholar]
- 130.Hang L, Blum AM, Setiawan T, et al. Heligmosomoides polygyrus bakeri Infection Activates Colonic Foxp3+ T Cells Enhancing Their Capacity To Prevent Colitis. J Immunol. 2013;191:1927–34. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hiemstra LH, Klaver EJ, Vrijland K, et al. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Molec Immunol. 2014;60:1–7. doi: 10.1016/j.molimm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 132.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 133.Kellermayer R. Prospects and challenges for intestinal microbiome therapy in pediatric gastrointestinal disorders. World J Gastrointest Pathophys. 2013;4(4):91–3. doi: 10.4291/wjgp.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russell G, Kaplan J, Ferraro M, et al. Fecal Bacteriotherapy for Relapsing Clostridium difficile Infection in a Child: A Proposed Treatment Protocol. Pediatrics. 2010;126(1):e239–42. doi: 10.1542/peds.2009-3363. [DOI] [PubMed] [Google Scholar]
- 135.Kunde S, Pham A, Bonczyk S, et al. Safety, Tolerability, and Clinical Response After Fecal Transplantation in Children and Young Adults With Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2013;56(6):597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 136.Vandenplas Y, Veereman G, van der Werff ten Bosch J, et al. Fecal microbial transplantation in a one-year-old girl with early onset colitis- caution advised. J Pediatr Gastroenterol Nutr. 2014 doi: 10.1097/MPG.0000000000000281. In Press. [DOI] [PubMed] [Google Scholar]
- 137.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]