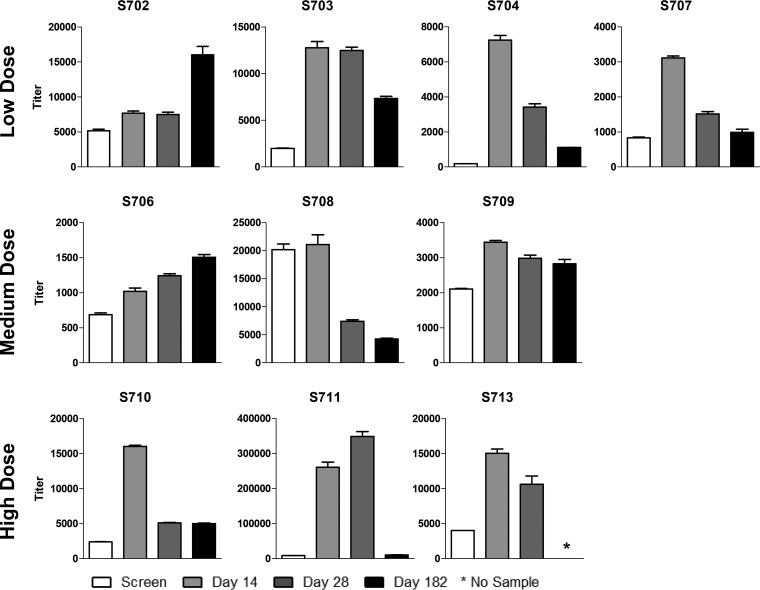
FIG 2.
Analysis of human parainfluenza virus type 1 (hPIV-1)-specific antibody among recipients of intranasal SeV vaccine. Serum samples obtained from ten 3- to 6-year-old participants at indicated time points prevaccination and postvaccination were examined for hPIV-1-specific antibody binding. Results are reported as antibody titers defined by nonlinear regression calculations. Antibody binding titer was defined as the highest sample dilution that scored an OD of at least 0.1 at 405 nm on the ELISA. Means and standard errors are shown. *, assays were conducted prior to collection of the 6-month sample from participant S713. Student's t tests were performed to compare hPIV-1-specific binding antibody responses before and after vaccinations. Except for one participant (S708), significant improvements in hPIV-1-specific antibody binding titers occurred on day 14 after vaccinations compared to prevaccination titers for all individuals (Student's t test [GraphPad Prism], P < 0.05).