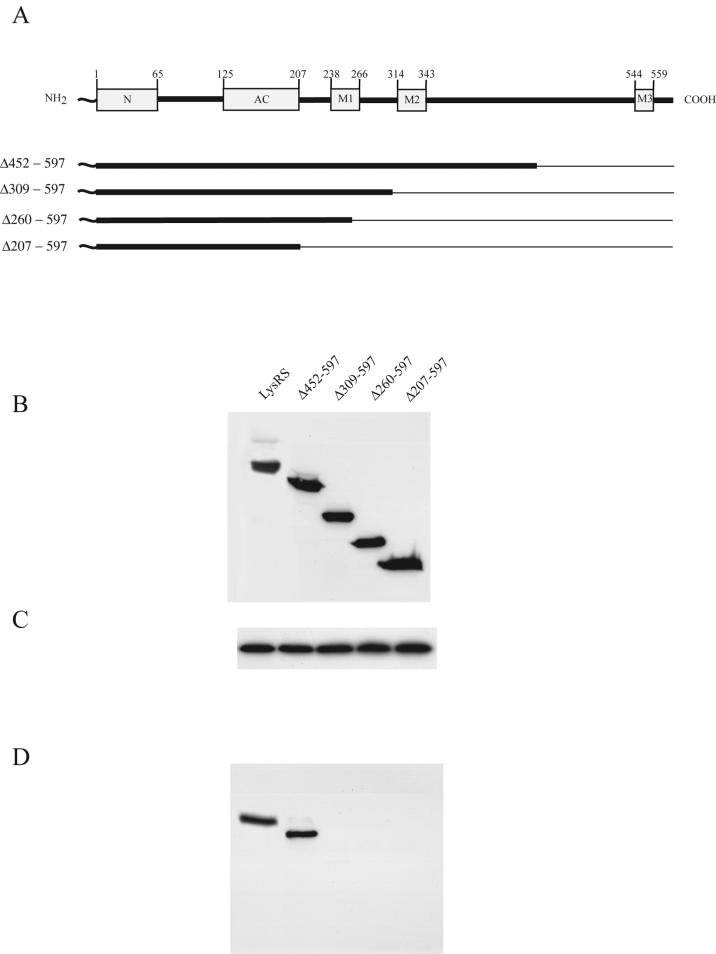
FIG. 4.
Interaction of wild-type or mutant LysRS with p38. Plasmids coding for wild-type or C-terminal deletion mutant LysRS tagged at the N terminus with Myc were transfected into 293FT cells. (A) Wild-type and mutant LysRS variants tested. The cartoon at the top shows the various LysRS domains and the amino acid positions (numbers) at which they occur. The unnumbered N-terminal squiggle represents Myc. Deleted amino acid sequences are shown graphically as thin lines and are listed to the left of each mutant. The N-terminal domain (N), the anticodon binding domain (AC) and motifs 1, 2, and 3 (M1, M2, and M3) are sequence elements characteristic of class II tRNA synthetases and are associated with tRNA binding (N and AC), LysRS dimerization (M1), and aminoacylation (M2 and M3). (B and C) Western blots of lysates of cells transfected with plasmids coding for the different LysRS species. Blots were probed with either anti-Myc (B) or anti-p38 (C). (D) Cell lysates were treated with anti-p38, and the p38 immunoprecipitate was analyzed by Western blots probed with anti-Myc.