Figure 2. Chemical generated ROS schemes.
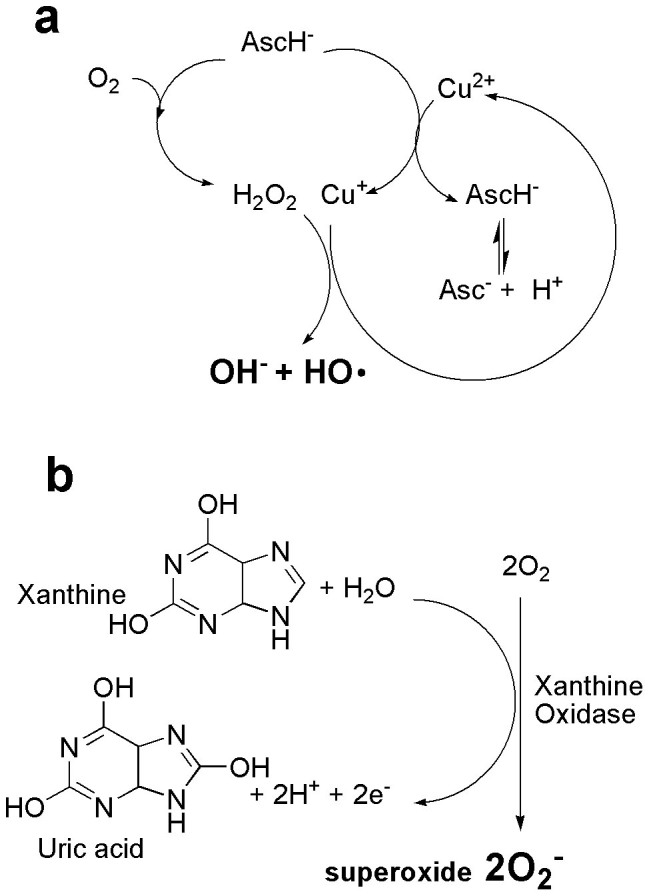
(a) Formation of hydroxyl radicals (HO·) via Fenton reaction [CuSO4, phenanthroline, and ascorbic acid; CPA]. Under aerobic conditions, ascorbate (AscH−) not only is involved in the reduction of copper ions (Cu2+), but also reacts with O2 to produce H2O2. Hydroxide (OH−) and HO· are then yielded in the next Fenton reaction. 1, 10-phenanthroline (P) is used to stimulate HO· formation with Cu2+ ions and AscH− (b) Formation of superoxide anion (O2−) by xanthine (1 mM) plus xanthine oxidase (0.05 U/ml). Xanthine (X) is catalyzed by xanthine oxidase (XO) enzyme and form uric acid and also generates O2− in this reaction. This mechanism is based on proposal that the one-electron transfer equilibriums between redox centers of XO enzymes (one molybdenum, one FAD, and two Fe-S centers) are rapid and governed by reduction potentials. During the oxidation of reduced XO electrons transferred to dioxygen. Two O2− are produced for each enzyme molecules reoxidized.
