Abstract
In Drosophila, prior fighting experience influences the outcome of later contests: losing a fight increases the probability of losing second contests, thereby revealing “loser” effects that involve learning and memory. In these experiments, to generate and quantify the behavioral changes observed as consequences of losing fights, we developed a new behavioral arena that eliminates handling. We compared two commonly used fly handling procedures with this new chamber and demonstrated that handling influences aggressive behavior and prevents “loser” effect formation. In addition, we induced and observed novel aspects of learning associated with aggression such as the formation of robust winner effects.
A critical adaptive trait for animals is the ability to learn from past experience and modify subsequent behavior in a constantly changing sensory landscape. Learning and memory studies using molecular, biochemical, and neurophysiological tools have been particularly successful in “simple systems” (Castellucci and Kandel 1976; Rankin et al. 1990). Among these, Drosophila melanogaster stands out as a powerful model system in which sophisticated genetic tools can be used to unravel the roles of genes and neural circuits in complex behaviors including learning and memory. In early studies, an operant conditioning paradigm associated with olfaction was used to study learning and memory in Drosophila (Quinn et al. 1974); later a classical conditioning paradigm was used due to the higher learning index scores obtained (Tully and Quinn 1985). These studies demonstrated that a strong association exists between the training paradigm and memory consolidation and retention.
Flies exhibit a variety of social behaviors, including courtship (Pavlou and Goodwin 2013), aggression (Chen et al. 2002), and aggregation (Simon et al. 2012), in which learning and memory are believed to be involved. Of these, aggression is found across the animal kingdom and is used to acquire resources, like food, territory, and mates via the establishment of social hierarchies. The first report on aggressive behavior in flies was by Sturtevant (1915) followed by further studies in which multiple animals were present in fighting arenas and aggression was scored over extended periods of observation (Dow and von Schilcher 1975; Hoffmann 1987). Later, to reduce the complexity of the experiments and to simplify the interpretation of results, Chen et al. (2002) paired only two males in arenas for shorter periods of time.
At present, many laboratories are studying aggression using the Drosophila model. Different experimental protocols, however, are being used in the various laboratories. Most of them utilize gentle aspiration to introduce pairs of males to the behavioral chambers (Dierick 2007; Wang et al. 2011; Alekseyenko et al. 2013). In some studies, flies are anesthetized prior to introducing them to arenas by using a brief dip of their isolation vials into an ice-water bath (Williams et al. 2014), or by using CO2 anesthesia (Yuan et al. 2014). Since many studies have reported detrimental effects of CO2 anesthesia on courtship, flight, wing movement behaviors, and on lifespan, we do not consider that further here (Gilberts 1981; Gotz and Biesinger 1985; Joachim and Curtsinger 1990). Surprisingly, phototaxis and negative geotaxis, both innate fly behaviors, have been used in only one experimental protocol to introduce animals to experimental arenas (Liu et al. 2011).
The present studies are aimed at determining the effects of handling both on social behavior and on the learning and memory associated with aggression. Toward that end, we designed a new behavioral chamber that eliminates handling of flies by aspiration or cold anesthesia, and instead, uses negative geotaxis to introduce flies into fight arenas (see accompanying Supplemental Movie 1 and Supplemental Fig. 1A). Using these chambers, we compared fight dynamics and outcomes of 20-min bouts between pairs of male flies under different handling conditions. In all experiments, the controls are flies introduced into fighting chambers by negative geotaxis, while the experimentals are flies introduced to chambers either by gentle aspiration or by brief ice-bath anesthesia.
The number of encounters (brief meetings between the flies) was not reduced significantly in the three different conditions, but in both experimental groups a downward trend in encounter number was observed (Fig. 1A). In contrast, the total number of lunges (indicator of higher-intensity encounters—important in decision making) was significantly decreased in both experimental groups compared with the controls (Fig. 1B). In the aspiration group, despite significantly reduced numbers of lunges, the latency to lunge (time between the first encounter and the first lunge, Fig. 1C), and the numbers of encounters before the first lunge (Fig. 1D) were not significantly different from controls. This suggests that gentle aspiration does not alter the behavior of flies in the initial phases of fights but ultimately does alter their levels of aggressiveness. In the anesthesia group, however, the latency to lunge and the numbers of encounters before first lunge were significantly increased compared with the control and aspiration groups (Fig. 1C,D). Thus, cold anesthesia has significant negative effects on aggression, altering both the dynamics of the initiation of higher-intensity encounters between male flies and their levels of aggressiveness.
Figure 1.
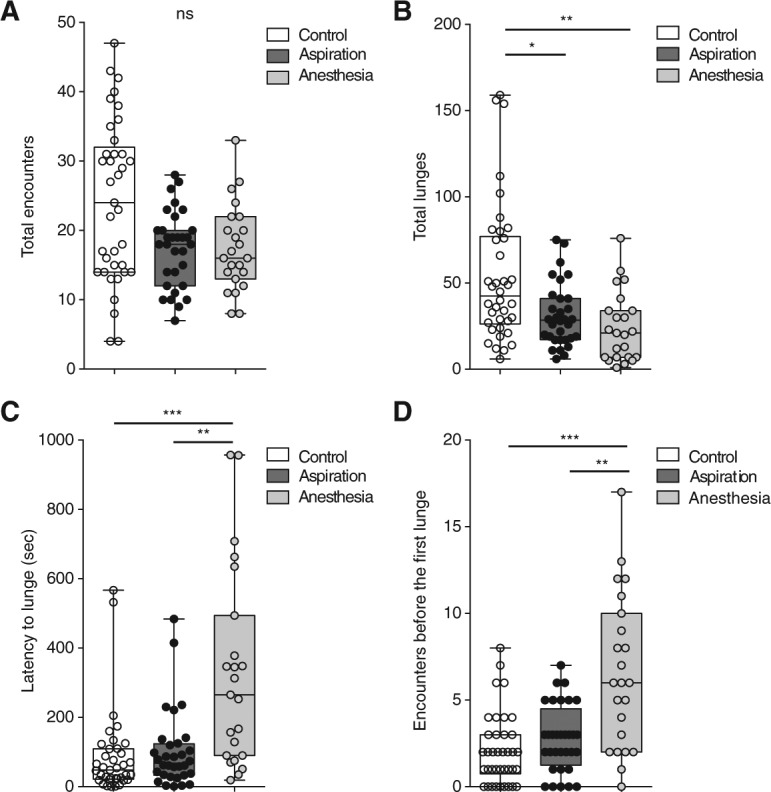
Pretest handling affects aggressive behavior. (A) The total number of encounters is not significantly different between control and experimental groups (Kruskal–Wallis statistic 7.24, Dunn's multiple comparison test; P > 0.05 n > 23). (B) The total number of lunges is significantly decreased for both experimental groups compared with the control (Kruskal–Wallis statistic 13.76 Dunn's multiple comparison test control/aspiration and control/anesthesia P < 0.05; n > 23). (C) The latency to lunge is significantly different between anesthesia and both control and aspiration groups (Kruskal–Wallis statistic 20.31, Dunn's multiple comparison test control/aspiration P > 0.05; control/anesthesia and aspiration/anesthesia P < 0.05; n > 23). (D) The number of encounters before the first lunge is significantly increased between anesthesia and both control and aspiration groups but not between aspiration and control groups (Kruskal–Wallis statistic 20.14, Dunn's multiple comparison test control/aspiration P > 0.05; control/anesthesia and aspiration/anesthesia P < 0.05; n > 23). Outliers were tested with a Grubb's test and removed.
Since cold anesthesia treatment leads to an increased latency to lunge, that might explain the decreased total number of lunges observed during a 20-min time period. To offset this possibility, we established an aggression vigor index (AVI) [see Krstic et al. 2009 for use of courtship vigor index rather than courtship index] that represents the numbers of lunges during 10 min after the first lunge. The AVI is significantly decreased in both experimental groups (Fig. 2A), confirming that pretest handling alters fly aggressiveness. Next, we compared the latencies to establish hierarchical relationships in the different groups (time between the first encounter and the establishment of dominance). Dominance is defined as the time when one fly (the “loser”) retreats three consecutive times from the food cup after receiving lunges from the “winner.” We observed that aspiration does not significantly alter the latency to establish dominance compared with controls. As anticipated from the previous results (Fig. 1C), however, the time between the first encounter and the establishment of dominance is increased in the anesthesia group (Fig. 2B). To ask whether the phenotypes observed in the experimental groups were caused by a shift in the beginning of fights, we compared the time with the first encounter (time between the beginning of the recording, after removing the divider from the fighting chamber, and the first encounter). This parameter is not significantly different between the three groups (Fig. 2C), although there is a wide distribution in times for each group, suggesting that handling does not alter the ability of flies to interact with each other. Together, these results demonstrate that handling flies before experiment, particularly cold anesthesia, is not a desirable handling procedure for the study of aggressive behavior in fruit flies.
Figure 2.
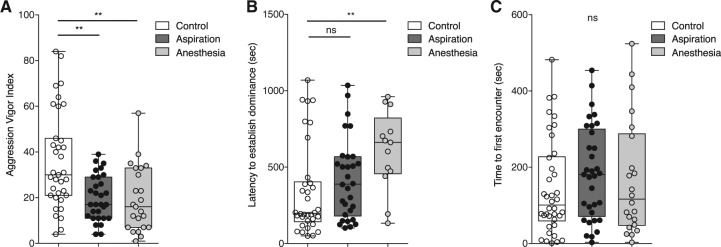
Pretest handling influences fight dynamics. (A) The aggression vigor index (AVI) is the number of lunges observed during the 10 min after the first lunge. This measure is significantly decreased in both experimental groups compared with the control (Kruskal–Wallis statistic 14.43, Dunn's multiple comparison test control/aspiration and control/anesthesia P < 0.05; n > 23). (B) The latency to establish dominance is not significantly different between the aspiration and control groups, but a significant difference is observed between anesthesia and control groups (Kruskal–Wallis statistic 9.03, Dunn's multiple comparison test control/aspiration P > 0.05 and control/anesthesia P < 0.05; n > 13). (C) The time to the first encounter is not significantly different between the three groups (Kruskal–Wallis statistic 2.01, Dunn's multiple comparison test control/aspiration and control/anesthesia P > 0.05; n > 22). Outliers were tested with a Grubb's test and removed.
We next asked whether handling alters other social behaviors like courtship. The courtship ritual of fruit flies is composed of a series of sequential actions that have been carefully and quantitatively described (Markow and Hanson 1981; Yamamoto et al. 2014). To study courtship, most experimental protocols require gentle aspiration to introduce pairs of male and female flies to courtship chambers (Goodwin and O'Dell 2012; Coen et al. 2014). On examining the latency to court, the latency to copulate and the courtship vigor index (CVI) in controls and the aspiration group, we found no statistically significant differences between the procedures (Supplemental Fig. 2A–C). While pretest handling by aspiration has only moderate effects on aggressive behavior (decreased total number of lunges and AVI), this procedure does not affect the ability to court. That might be explained by the robustness of this innate behavior observed across the animal kingdom.
Finally, we asked whether pretest handling influences locomotion by placing single flies into behavioral chambers, and counting the numbers of midline crossings over 20 min. Surprisingly, both the aspiration and anesthesia groups displayed significant reductions in locomotion (Supplemental Fig. 1B). To ask whether this was only a short-term transient effect of the handling procedures, we counted the numbers of midline crossings in 5-min time bins and noted the same reduced levels of locomotion throughout the entire 20 min of the test (Supplemental Fig. 1C). Thus, pretest handling leads to locomotion deficits in single flies. However, since the total number of encounters in fights (Fig. 1A) and courtship behavior (Supplemental Fig. 2) was not decreased significantly in experimental groups, we hypothesized that the presence of a second fly may be a sufficient stimulus to overcome any locomotion deficits caused by handling. By performing the same locomotion assay during 5 min with a group of 10 flies, we confirmed the hypothesis; the locomotion deficit previously observed with a single fly handled by aspiration was overcome in the presence of other flies (Supplemental Fig. 1D). However, the procedure using cold anesthesia as a pretest handling still causes a significant reduction in locomotion compared with both control and aspiration groups (Supplemental Fig. 1D). Together, these locomotion results reinforce the previous conclusions: gentle aspiration has only moderate effects while cold anesthesia leads to important detrimental consequences on social behavior.
In many species, prior fighting experience influences the outcome of later contests: winning or losing a previous fight strongly increases the probability of winning or losing the next contest (for review, see Hsu et al. 2006). In Drosophila, after hierarchical relationships have been established, a loser-like mentality develops in defeated flies that likely involves learning and memory (Yurkovic et al. 2006; Penn et al. 2010). In most cases, one fly (the winner) lunges more and retreats less while the other (the loser) does not lunge at all and instead retreats following each attack. This change in fighting behavior illustrates the capability of flies to modify and adapt their behavior in response to prior social experience.
In the next set of experiments, we asked if handling flies might alter the “loser mentality” displayed by losing flies in second contests. Since cold anesthesia has already been reported to disrupt short-term memory in Drosophila (Quinn and Dudai 1976; Tully et al. 1990), we did not include this treatment in these studies: instead we compared the effects of gentle aspiration against a control group. The experimental protocol was to introduce pairs of flies into fighting chambers by negative geotaxis (controls) or by gentle aspiration (experimental group) and allow them to interact for 20 min. Then, in controls, the flies were separated by inserting a thin opaque plastic divider into the fighting chamber, which was then removed 10 min later allowing the flies to interact for another 20-min period (Supplemental Movie 1). In the experimental group flies were placed back in their original vials by gentle aspiration between the two fights and reintroduced to the fight arenas again using aspiration.
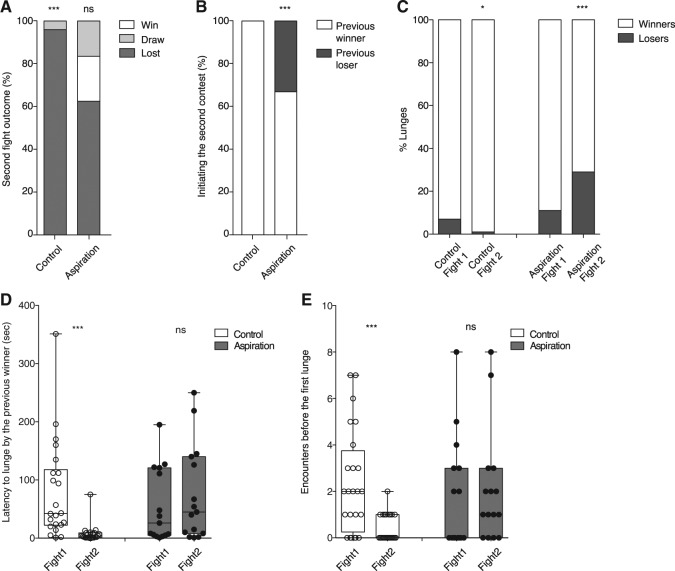
Figure 3A shows that 96% of previously defeated control flies lost their second fights, but only 63% of the previous losers lost their second fights in the experimental group. Fight outcomes were compared with an expected value of 50–50 in a two-tailed χ2 analysis and the loser effect is not significant in the experimental group. To explain the absence of a significant loser effect, we hypothesized that handling flies disrupts the adaptive behavioral changes in fighting strategy observed in second fights. In controls, in accord with previous studies, previous winners initiated all second contests (Fig. 3B). In the experimental group, however, only 67% of the previous winners initiated the second contests (Fig. 3B). By comparing the total numbers of encounters and lunges in first and second fights, we observed a significant decrease of these two parameters in both groups for the second fight (Supplemental Fig. 3A,B). By analyzing the numbers of lunges displayed by winners and losers separately in the two fights, significant differences were found. In the control group, losers were responsible for 9% of the lunges in first fights and only 1% in second fights (Fig. 3C). In the experimental group, however, the percentages of lunges displayed by losers changed from 11% in first fights to a surprising 29% in second fights (Fig. 3C). We next examined the latency to lunge by previous winners and the numbers of encounters before the first lunge in both fights. In controls, these parameters were significantly reduced in second fights (Fig. 3D,E) as expected along with the development of a loser mentality. In the experimental group, no significant differences were observed in either of these parameters in second fights (Fig. 3D,E). Thus, these results demonstrate that handling disrupts the normal behavioral adaptations of flies observed as a consequence of losing fights, thereby generating significant reductions in the magnitude of the “loser” effect. By separately analyzing the behavior of both winners and losers in first and second fights in the control group, however, we also could demonstrate a clear “winner” effect for the first time along with the previously established “loser” effect.
Figure 3.
“Loser/winner mentality” formation is disrupted by handling. (A) A significant loser effect (percentage of previous losers losing a second fight) is observed in the control group (lost: 96%, draw: 4%; χ2 test, P = 0.0001; n = 24). But, no significant loser effect is observed in the experimental group (lost: 62.5%, draw: 16.5%, win: 21%; χ2 test, P = 0.127; n = 24). (B) One hundred percent of previous winners deliver the first lunge in the second fight in the control group but only 67% do so in the experimental group (χ2 test, P < 0.0001; n = 24). (C) In the control group, winners deliver 93% of the total lunges in the first fight and 99% in the second. Previous losers deliver significantly fewer lunges in the second compared with the first fight (χ2 test, P = 0.019; n = 24). In the aspiration group, winners deliver 89% of the total lunges in the first but only 71% of the total lunges in the second fight. Losers show 11% of the lunges in the first and 29% in the second fight. In contrast to controls, previous losers in the aspiration group display a significantly increased number of lunges in second fights compared with first fights (χ2 test, P < 0.0001; n = 24). (D) The latency to lunge by previous winners is significantly decreased between the first and second fights in the control (Wilcoxon matched pairs test, P < 0.0001; n = 22) but not in the experimental group (Wilcoxon matched pairs test, P = 0.359; n = 15). (E) A significant decrease in the numbers of encounters before the first lunge is observed between the two fights in the control group where the previous winners lunge first in second fights (Wilcoxon matched pairs test, P < 0.0001; n = 24), but not in the experimental group (Wilcoxon matched pairs test, P = 0.7778; n = 15). Outliers were tested with a Grubb's test and removed.
In summary, we developed a new general-purpose experimental chamber that can be used for studying social behavior. The design of this chamber allows loading of flies by natural negative geotaxis and eliminates any handling after the pupal stage. Using this chamber, we explored the effects of two types of handling routinely used in laboratories to study social behaviors. We found that cold anesthesia profoundly reduces aggressive behavior. The latencies to lunge and to establish hierarchical relationships are increased, and the general aggressiveness of flies is decreased. Furthermore, our results are consistent with a previous study demonstrating that considerable reductions were observed in courtship and aggression by cold-treating Drosophila grimshawi males (Ringo 1971). Cold anesthesia also resulted in the most significant reductions in locomotor activity of all conditions tested. We also showed that gentle aspiration has no effects on courtship behavior and only moderate effects on aggressive behavior. However, aspiration does have important effects on the learning process during first fights and/or on the memory consolidation during the period of rest, both necessary for “loser” and “winner” effect formation among combatants after hierarchical relationships are established.
Loser and winner effects have been widely studied in many species, including insects (Stevenson and Rillich 2013), fish (Chase et al. 1994), and mammals (Schwartzer et al. 2013). Few studies, however, have reported such effects on Drosophila (Yurkovic et al. 2006; Penn et al. 2010). With this model system, a “loser mentality” originally was demonstrated in a larger chamber than the one used in this study. In addition, male flies were pretest handled and longer fighting periods with greater interval times between fights were required (Yurkovic et al. 2006; Penn et al. 2010). In the present study, where flies were not handled prior to experiments, we showed that clear hierarchical relationships could be established after only 20 min. This proved to be a sufficient time to induce changes in fighting strategies in first fights and to establish robust “loser” and “winner” effects observable in second fights. Importantly, a “winner” effect has not been reported before in Drosophila. Here, we found that prior winners are more likely to initiate second contests, lunge more rapidly with the first lunge commonly seen during the first encounter, and win significantly more of their second contests.
As with other organisms, Drosophila behavior is strongly influenced by environmental factors. Stressful situations may compromise the ability of flies to adapt to rapidly changing environments, thereby putting them at a disadvantage in competition with others for survival, resources, and mates. In the present study, by eliminating handling, we revealed aspects of behavior that were previously concealed by experimental manipulations. We also found that we could incorporate learned behavioral traits into behavioral repertoires more reliably.
Supplementary Material
Acknowledgments
We thank all the members of the Kravitz Laboratory for many helpful discussions and support on this project. This research was supported by grants from the National Institute of General Medical Sciences (GM0067645 and GM074675) to E.A.K. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: S.T., B.C., and E.A.K. designed the behavioral chamber. S.T. designed the experiments. S.T. and B.C. performed and analyzed experiments. S.T., E.A.K., and B.C. wrote the paper.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.036418.114.
References
- Alekseyenko OV, Chan YB, Li R, Kravitz EA 2013. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci 110: 6151–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V, Kandel ER 1976. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science 194: 1176–1178. [DOI] [PubMed] [Google Scholar]
- Chase ID, Bartolomeo C, Dugatkin LA 1994. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim Behav 48: 393–400. [Google Scholar]
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA 2002. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci 99: 5664–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen P, Clemens J, Weinstein AJ, Pacheco DA, Deng Y, Murthy M 2014. Dynamic sensory cues shape song structure in Drosophila. Nature 507: 233–237. [DOI] [PubMed] [Google Scholar]
- Dierick HA 2007. A method for quantifying aggression in male Drosophila melanogaster. Nat Protoc 2: 2712–2718. [DOI] [PubMed] [Google Scholar]
- Dow MA, von Schilcher F 1975. Aggression and mating success in Drosophila melanogaster. Nature 254: 511–512. [DOI] [PubMed] [Google Scholar]
- Gilberts DG 1981. Effects of CO2 vs. ether on two mating behavior components of D. melanogaster. Drosophila Info Serv 56: 45–46. [Google Scholar]
- Goodwin SF, O'Dell KM 2012. The best laid plans: analyzing courtship defects in Drosophila. Cold Spring Harb Protoc 2012: 1140–1145. [DOI] [PubMed] [Google Scholar]
- Gotz KG, Biesinger R 1985. Centrophobism in Drosophila melanogaster. I. Behavioral modification induced by ether. J Comp Physiol A 156: 319–327. [Google Scholar]
- Hoffmann A 1987. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim Behav 35: 807–818. [Google Scholar]
- Hsu Y, Earley RL, Wolf LL 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 81: 33–74. [DOI] [PubMed] [Google Scholar]
- Joachim D, Curtsinger JW 1990. Genotype and anesthetic determine mate choice in Drosophila melanogaster. Behav Genet 20: 73–79. [DOI] [PubMed] [Google Scholar]
- Krstic D, Boll W, Noll M 2009. Sensory integration regulating male courtship behavior in Drosophila. PLoS One 4: e4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y 2011. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci 14: 896–902. [DOI] [PubMed] [Google Scholar]
- Markow TA, Hanson SJ 1981. Multivariate analysis of Drosophila courtship. Proc Natl Acad Sci 78: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou HJ, Goodwin SF 2013. Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr Opin Neurobiol 23: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JK, Zito MF, Kravitz EA 2010. A single social defeat reduces aggression in a highly aggressive strain of Drosophila. Proc Natl Acad Sci 107: 12682–12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Dudai Y 1976. Memory phases in Drosophila. Nature 262: 576–577. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S 1974. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci 71: 708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, Chiba CM 1990. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res 37: 89–92. [DOI] [PubMed] [Google Scholar]
- Ringo JM 1971. The effects of anesthetization upon survival and behavior of D. grimshawi. Drosophila Info Serv 47: 118–119. [Google Scholar]
- Schwartzer JJ, Ricci LA, Melloni RH Jr 2013. Prior fighting experience increases aggression in Syrian hamsters: implications for a role of dopamine in the winner effect. Aggress Behav 39: 290–300. [DOI] [PubMed] [Google Scholar]
- Simon AF, Chou MT, Salazar ED, Nicholson T, Saini N, Metchev S, Krantz DE 2012. A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav 11: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Rillich J 2013. Isolation associated aggression—a consequence of recovery from defeat in a territorial animal. PLoS One 8: e74965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH 1915. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Animal Behav 5: 351–366. [Google Scholar]
- Tully T, Quinn WG 1985. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157: 263–277. [DOI] [PubMed] [Google Scholar]
- Tully T, Boynton S, Brandes C, Dura JM, Mihalek R, Preat T, Villella A 1990. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol 55: 203–211. [DOI] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ 2011. Hierarchical chemosensory regulation of male–male social interactions in Drosophila. Nat Neurosci 14: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Goergen P, Rajendran J, Klockars A, Kasagiannis A, Fredriksson R, Schioth HB 2014. Regulation of aggression by obesity-linked genes TfAP-2 and Twz through octopamine signaling in Drosophila. Genetics 196: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D, Sato K, Koganezawa M 2014. Neuroethology of male courtship in Drosophila: from the gene to behavior. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 200: 251–264. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Song Y, Yang CH, Jan LY, Jan YN 2014. Female contact modulates male aggression via a sexually dimorphic GABAergic circuit in Drosophila. Nat Neurosci 17: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovic A, Wang O, Basu AC, Kravitz EA 2006. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci 103: 17519–17524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.