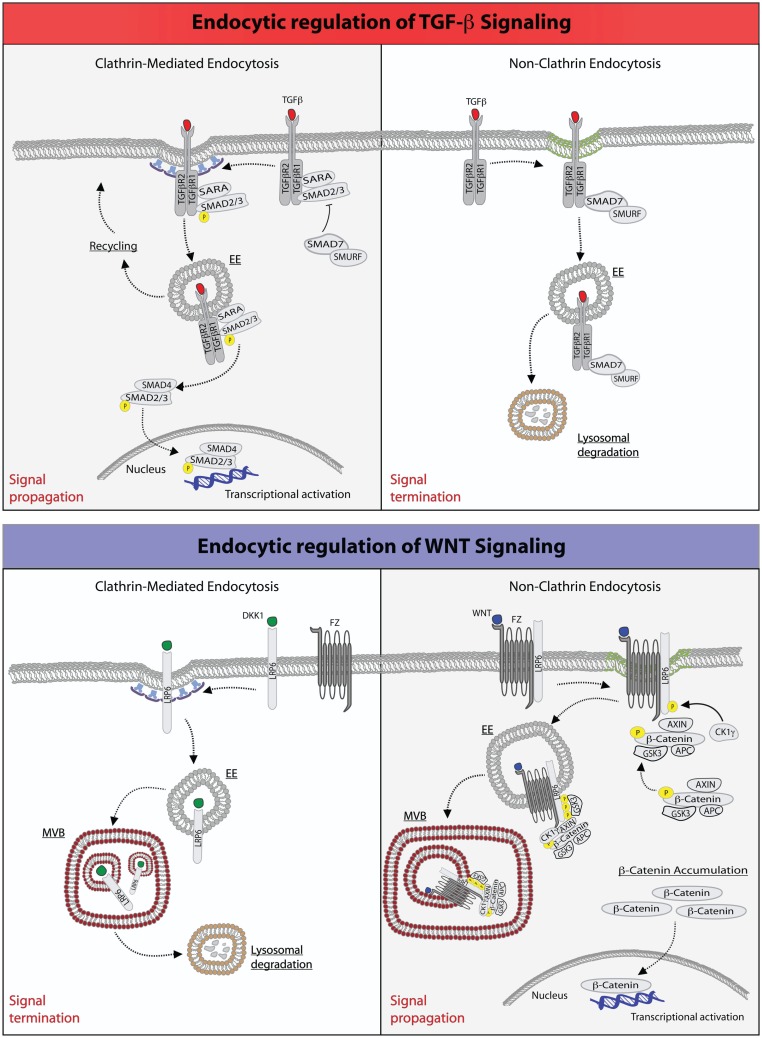
Figure 1.
Endocytic regulation of TGF-β and WNT pathways. Top: endocytic regulation of TGF-β signaling. Left panel, upon ligand stimulation, type-I and type-II TGF-β-receptors form a heterodimeric complex (30, 36–38), which binds the SMAD2/3 and SARA proteins. Type-I TGF-β-receptor directly phosphorylates SMAD2/3, an event that may be promoted by the anchoring protein SARA (41–43, 50). SARA, in addition to facilitate SMAD2/3 phosphorylation at the PM, may also retain these proteins at this location retarding their release into the cytoplasm. Ligand-bound TGF-β-receptor is also rapidly internalized via CME into early endosome (EE) (41, 50). From this endocytic station, the receptor can be recycled back to the plasma membrane for a new round of signaling. Early endosomes, where SARA accumulates, may also serve as a mean to separate SARA from activated SMAD2/3, which would then be free to be released into the cytoplasm, where they form an oligomeric complex with SMAD4. The SMAD2/3-SMAD4 complex translocates, then, into the nucleus where it acts as a transcription factor ensuring execution of TGF-β signaling. Right panel, activation of TGF-β receptor(s) may also occur in cholesterol-rich membranes micro-domains (green membrane) (41, 50). This promotes the binding to the receptors of the SMAD7-SMURF2 complex followed by internalization into caveolae (51, 52). SMURF2 is an ubiquitin ligase that can ubiquitinate the receptor promoting its targeting into multivesicular bodies and its subsequent lysosomal degradation resulting in signal termination (51, 52). Bottom: endocytic regulation of WNT signaling. Left panel, upon binding of the antagonistic, WNT-like ligand, Dickkopf (DKK1), the low-density lipoprotein receptor-related 6 (LPR6) receptor is internalized by CME, and primarily transported through multivesicular body (MVB) to lysosomes for degradation, hence terminating signaling (33, 35). Right panel, the binding of WNT to the Frizzled (Fz) receptor, a seven-pass transmembrane receptor, promotes the formation of complex between Fz and LPR6 on cholesterol-rich lipid domain (green membrane). At this site, the cytoplasmic tail of LPR6 is phosphorylated by casein kinase 1γ (CK1γ) and glycogen synthase kinase 3 (GSK3), thereby activating NCE of the receptors (53). The components of the destruction complex – Axin, β-catenin, APC, and GSK3 – become bound to the activated and internalized Fz-LRP6 complex in endosomes. The subsequent transport of this assembly into MVBs may lead to the sequestration of the destruction complex into the internal vesicles of the MVB. Newly synthesized β-catenin may, thus, escape the destruction complex and accumulate into the cytoplasm to translocate into the nucleus and activate gene transcription (54).