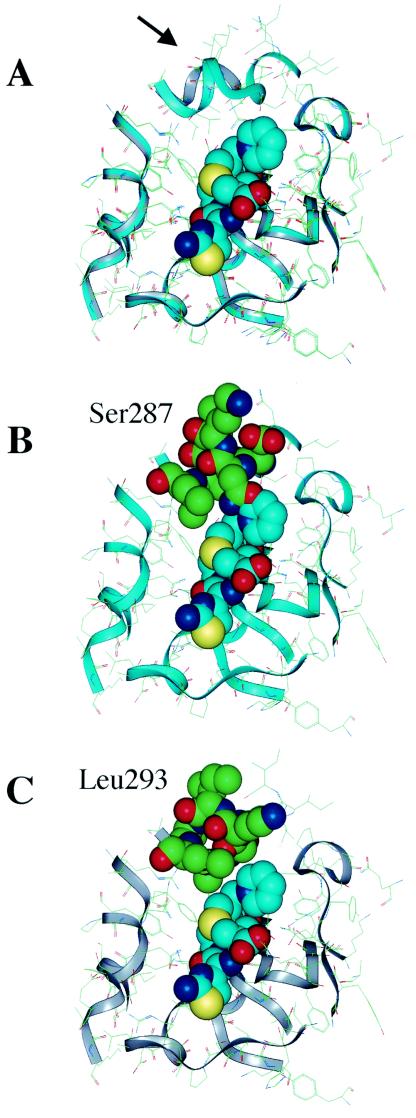
FIG. 2.
Optimized ribbon structures of ceftazidime docked in the active site of HKY28 AmpC (gray) compared with that of E. coli K-12 AmpC (light blue) (10). (A) The two structures are superimposed. The Gly286-Ser287-Asp288 deletion in the HKY28 AmpC creates an open space at the top of the binding site (arrow) that allows the accommodation of the R-2 side chain of ceftazidime in the E. coli K-12 AmpC and the R-2 side chain of ceftazidime collides with Ser287(B), but in the HKY28 AmpC (C) it comes near Leu293 but does not make direct contact.