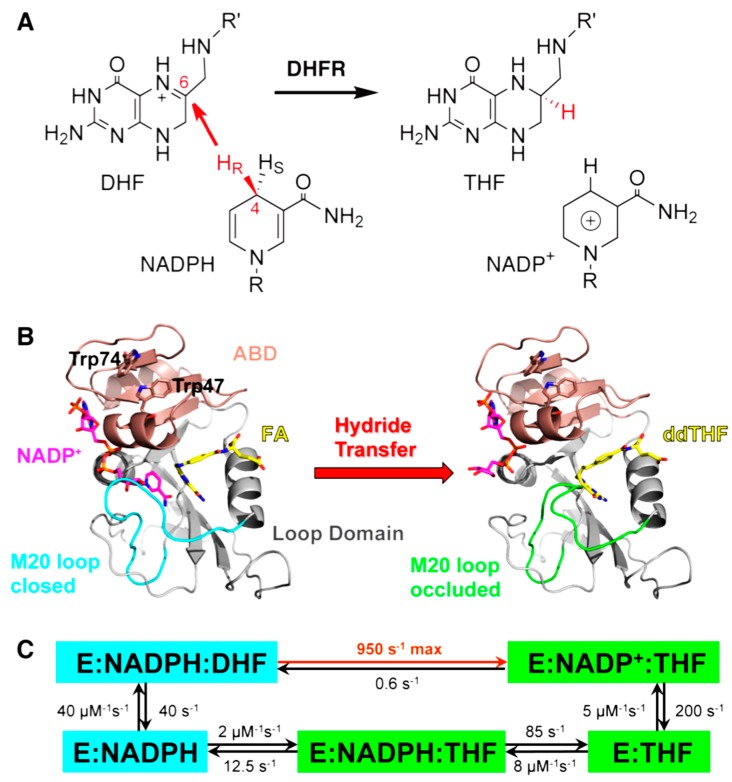
Figure 1.
(A) DHFR catalyzes the stereospecific transfer of the pro-R hydride of C4 on NADPH to C6 of protonated N5-DHF, producing THF and the oxidized cofactor NADP+. (B) The active site cleft of DHFR divides the protein into two domains: the adenosine binding domain (ABD, residues 38–88) binds the adenosine moiety of the cofactor NADPH, while the loop domain (~100 residues) is dominated by three loops surrounding the active site. The ternary complex of DHFR with NADP+ (magenta) and folic acid (FA, yellow) mimics the Michaelis complex (structure on the left, PDB code 1RX2). The M20 loop (cyan) closes over the active site to ensure close proximity of the hydride donor (C4 of NADPH) and acceptor (C6 of DHF). The ternary complex of DHFR with NADP+ (magenta) and 5,10-dideazatetrahydrofolic acid (ddTHF, yellow) mimics the product complex (structure on the right, PDB code: 1RX4): here the M20 loop (green) protrudes into the binding site of the nicotinamide ribose moiety of the cofactor to facilitate product release. (C) Under cellular conditions of E. coli (with abundant NADPH concentrations), DHFR cycles through 5 kinetic intermediates, which are colored according to their M20 loop conformations (cyan: closed; green: occluded). The rate constants of each step are from [18]. The maximum (pH-independent) hydride transfer rate (950 s−1) was obtained from non-linear regression of the pH dependence of observed rate constants (pH 5.5–9.0) in stopped-flow experiments [18]. Reprinted from [19] with permission from the American Chemical Society.