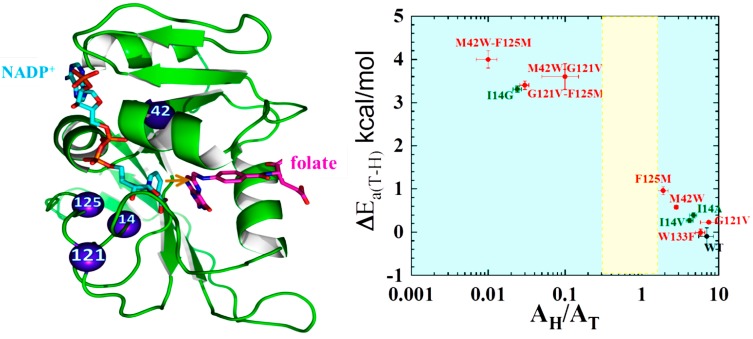
Figure 2.
Roles of active site and distal residues on the DHFR catalyzed reaction. Left panel: Structure of WT-DHFR (PDB Code 1RX2), with folate in magenta and NADP+ in light blue. A yellow arrow marks the hydride’s path from C4 of the nicotinamide to C6 of the folate, and the residues studied in ref [10] are marked as blue spheres. Right panel: Presentation of the temperature-dependence parameters of intrinsic KIE for WT (black), distal (red), and active site I14 (green) mutants of DHFR, where error bars represent standard deviation. The ordinate is the isotope effect on the activation energy (ΔEa) and the abscissa is the isotope effect on the Arrhenius pre-exponential factor (AH/AT) both of which were determined from a non-linear regression of the intrinsic KIEs to the Arrhenius equation. The yellow block represents the semi-classical range of the Arrhenius pre-exponential factor (0.3–1.7) [49]. Reproduced from ref. [10] with permission from the American Chemical Society.