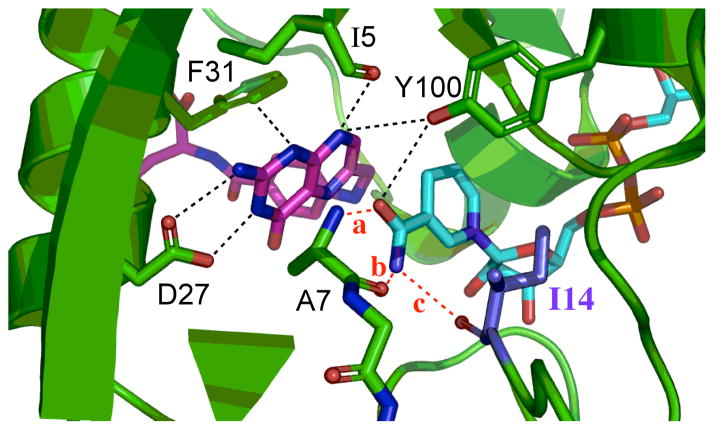
Figure 2.
The active-site of DHFR from E. coli (PDB ID 1RX2) emphasizing the role of Ile14 (metallic blue) as a support of the nicotinamide ring of NADP+. The nicotinamide ring is highlighted in light blue and the folate in magenta. Several other residues that form hydrogen bonding with the amide of NADPH are highlighted as well as I14 and A7. Three distinct hydrogen bonds, labeled in red, are: (a): NADPH(O-amide)-Ala-7(H); (b): NADPH(H72)-Ala-7(O); and (c): NADPH(H71)-Ile-14(O). The pterin ring is also immobilized in the active site via tight van der Waals interactions with F31, and strong hydrogen bonds to D27 and I5.