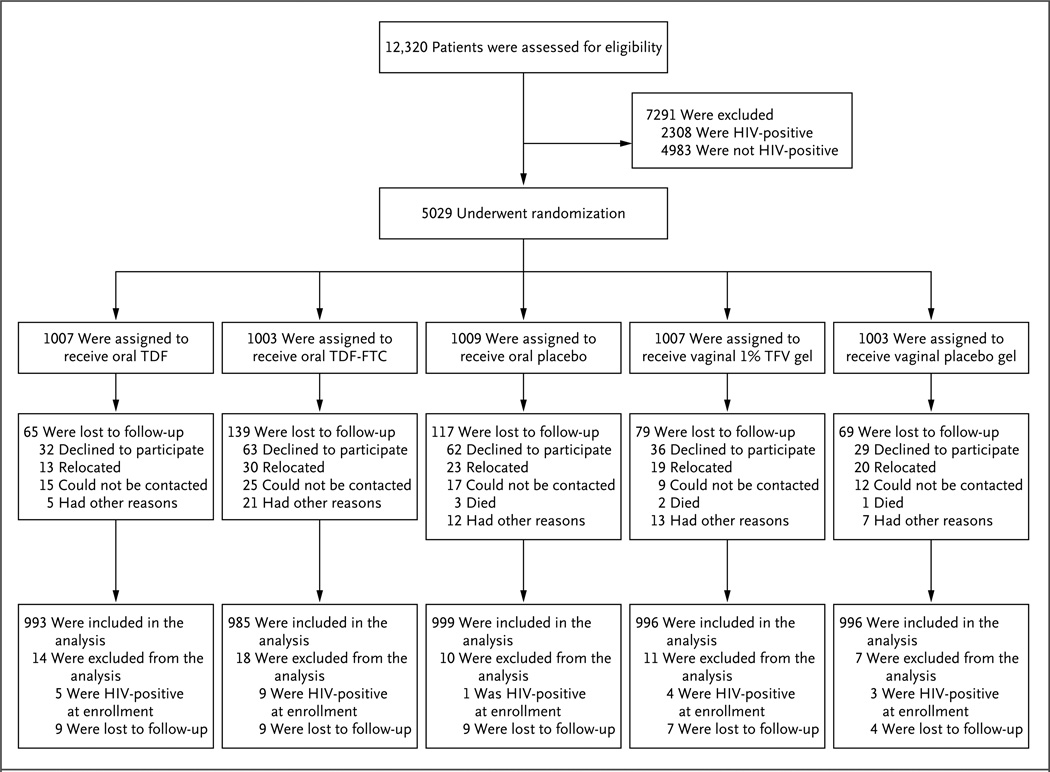
Figure 1. Study Cohort.
The most common reason for exclusion from participation was prevalent HIV infection (33% of excluded participants). Failure to complete screening and enrollment within the 56-day window resulted in the exclusion of 21% of potential participants. Collectively, abnormal laboratory results accounted for 16% (range, 5 to 23) of all exclusions; the most common results were positivity for the hepatitis B surface antigen (3.4%), anemia (2.9%), abnormal (grade 2 or higher) Papanicolaou smear (2.7%), and a urine-dipstick result of 2+ or higher for protein (2.5%). Conditions related to reproductive outcomes resulted in the exclusion of 9% of the women: 5.9% were pregnant at the time of screening, with the remainder reporting current breast-feeding or the intention to become pregnant in the next 2 years. A total of 22 participants who were found to have HIV-1 RNA, as assessed by means of a polymerase-chain-reaction assay, at the time of enrollment were classified as having acute HIV-1 infection and were excluded from the analysis. FTC denotes emtricitabine, TDF tenofovir disoproxil fumarate, and TFV tenofovir.