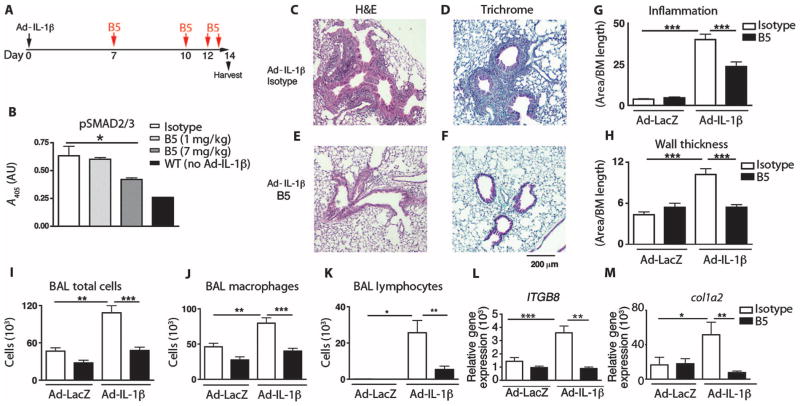
Fig. 1. Optimized B5 antibody blocks TGF-β activation in vivo and intratracheal Ad-IL-1β–induced airway inflammation and fibrosis.
(A) Schematic of the creation of a mouse model of airway inflammation using adenovirally delivered IL-1β (Ad-IL-1β) administered intratracheally. (B) B5 blocks intratracheal Ad-IL-1β–induced pSMAD2/3, demonstrating that neutralization of αvβ8 inhibits TGF-β activation in vivo. Lung homogenates from mice treated with B5, compared with IgG2a isotype or wild-type (WT) non–Ad-IL-1β injected intratracheally, were evaluated by pSMAD2/3 enzyme-linked immunosorbent assay (ELISA). n = 4, *P = 0.03 by analysis of variance (ANOVA) and post-test for linear trend. (C to H) B5 (D and F) compared with isotype control (C and E) blocks inflammation of the airway wall (C, D, and G) and fibrosis (E, F, and H) induced by intratracheally administered Ad-IL-1β. Results expressed as area of inflammation or fibrosis per basement membrane (BM) length. Semiquantitative airway morphometry of standard hematoxylin and eosin (H&E)–stained (C and D) or trichrome-stained (E and F) sections. ***P < 0.0001, by ANOVA and Tukey’s post-test. Scale bar, 200 μm. (I to M) B5 blocks intratracheal Ad-IL-1β–induced inflammation in bronchoalveolar lavage. Total cells in bronchoalveolar lavage (I), macrophages (J), and neutrophils (K), as well as gene transcripts of ITGB8 (L) and col1a2 (M), were increased by intratracheal Ad-IL-1β, and this increase was inhibited by B5. n = 3, Ad-LacZ+isotype– or Ad-LacZ+B5–treated mice; n = 4, Ad-IL-1β+isotype–treated mice; or n = 6, Ad-IL-1β+B5–treated mice. *P < 0.05, ** P < 0.01, ***P < 0.001, by ANOVA and Tukey’s post-test.