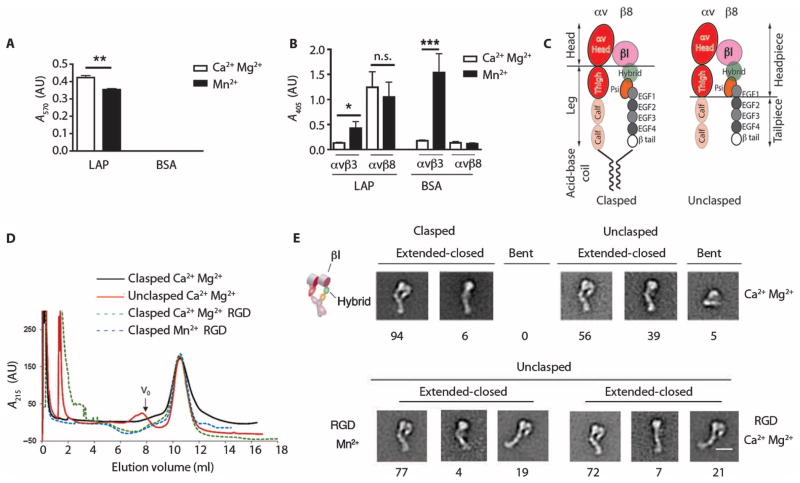
Fig. 3. Integrin αvβ8 is constitutively active in a high-affinity extended-closed conformation.
(A) Adhesion of β8-expressing 293 cells to latency-associated peptide (LAP) or bovine serum albumin (BSA; control), with Ca2+/Mg2+ or Mn2+, and reported as absorbance (A570). n = 3 experiments. (B) Binding of soluble αvβ8–alkaline phosphatase (AP) or αvβ3-AP fusion proteins to latency-associated peptide, or to the αvβ3 ligand fibronectin as a control, with Ca2+/Mg2+ (open bars) or Mn2+ (solid bars) reported as A405. n = 8 experiments. ***P < 0.001, by ANOVA and Tukey’s post-test. n.s., not significant. (C) Schematic of the domain structure of secreted integrins with or without a C-terminal clasp with locations of various domains. Clasped version has a 10–amino acid linker between the acid-base coil. (D) Size exclusion chromatography of clasped and unclasped αvβ8 secreted proteins. Clasped protein or unclasped protein in a solution containing Ca2+/Mg2+, unclasped protein with an RGD peptide in a solution containing Ca2+/Mg2+ or Mn2+. Particles too large to enter the medium are excluded and this volume is denoted as “void volume (V0)” as indicated. n = 3. (E) Negative staining electron microscopy of peak fractions shown in (D). Representative class averages showing extended-closed or bent conformations. Cartoon depicts domain structure. Below the micrographs are shown percentages of each subclass. Scale bar, 10 nm.