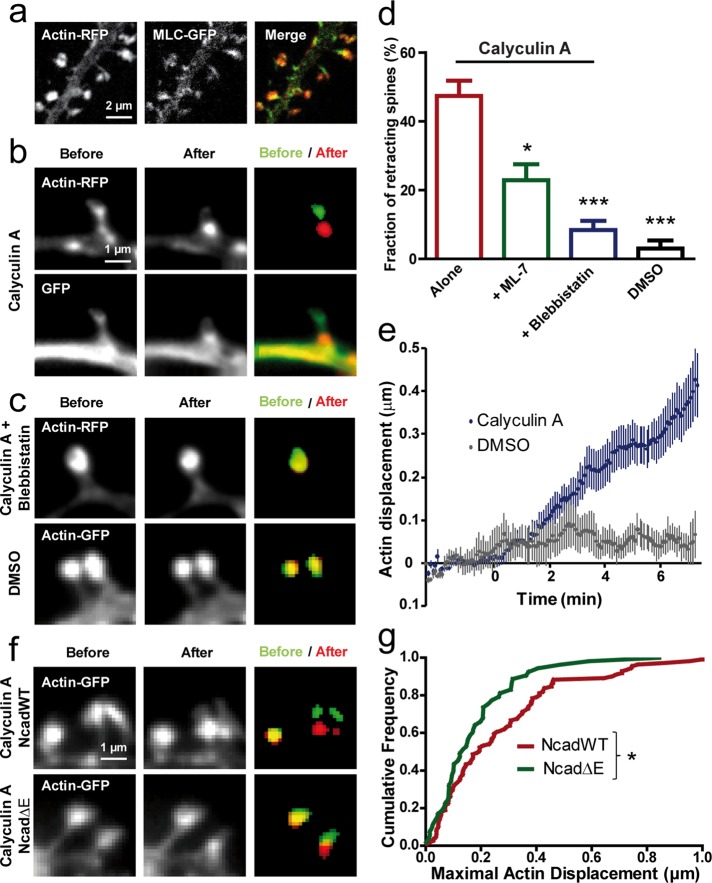
FIGURE 4:
Stimulating myosin contractility induces actin retraction in spines. (a) Neurons at DIV 17–21 coexpressing actin-RFP and MLC-GFP were dual-immunolabeled for GFP and RFP and examined by confocal microscopy. The merge image reveals the downstream localization of MLC-GFP compared with actin-RFP in spine heads. (b) Neurons coexpressing GFP and actin-RFP were imaged live in two colors and treated with calyculin A (1 μM). Note the retraction of spines in both channels. Segmented actin-GFP signal before (green) and at the end of the treatment (red) is represented on the merged image. (c) Neurons expressing actin-RFP were treated with calyculin A in the continuous presence of blebbistatin (50 μM), which blocked spine retraction. No retraction was detected either in spines expressing actin-GFP when the vehicle DMSO was added. (d) Fraction of retracting spines when calyculin A was added alone (178 spines, 7 neurons) or in the presence of ML-7 (279 spines, 6 neurons) or blebbistatin (357 spines, 9 neurons). As a control, DMSO was added alone (183 spines, 3 neurons; p < 0.0001 by one-way ANOVA). *0.01 < p < 0.05; ***p < 0.001. (e) Relative displacement of segmented actin-GFP spots over time. Neurons expressing actin-GFP were imaged every 5 s for 10 min, and calyculin A or the vehicle DMSO were added 2.5 min after the start of the recording (average ± SEM for 8 spines). (f) Representative actin-GFP images for cells coexpressing NcadWT or Ncad∆E, with similar calyculin A treatment as shown in b. (g) Cumulative distribution of the maximal displacement of actin-GFP spots within 10 min after drug addition for NcadWT or Ncad∆E conditions. Data were analyzed by nonparametric Mann–Whitney test. *0.01 < p < 0.05.