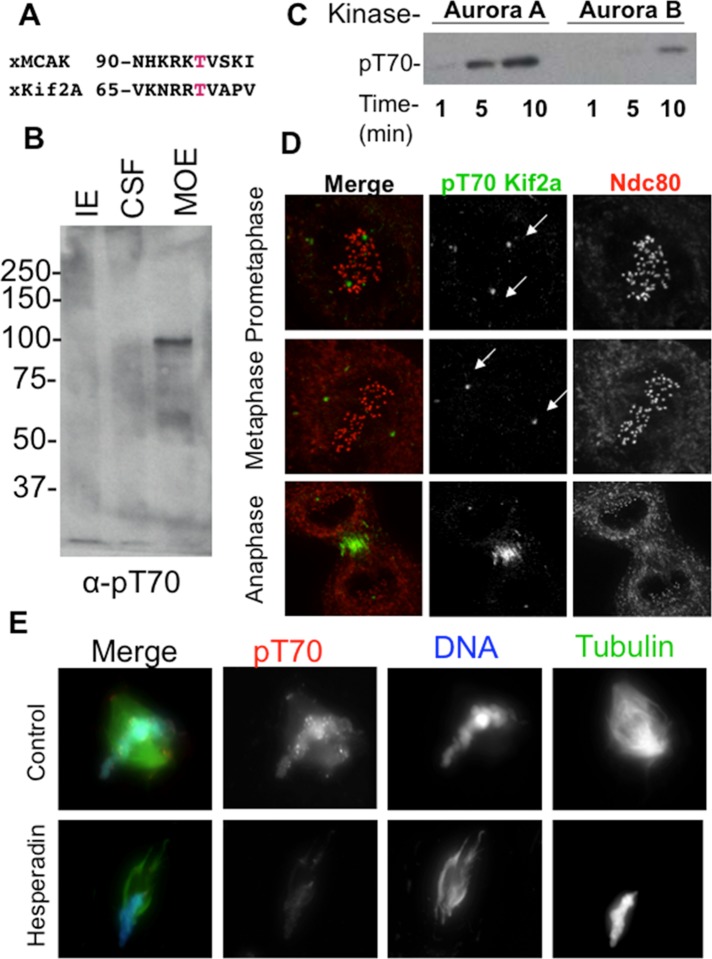
FIGURE 1:
Aurora kinases phosphorylate Kif2a on T70. (A) Alignment of putative Aurora consensus sites on Kif2a with their homologous MCAK phosphorylation sites. The putative phosphorylated residue is shown in red. (B) Immunoblot of anti-pT70 Kif2a antibody against three Xenopus extracts. CSF, cytostatic factor–arrested egg extract; IE, interphase extract; MOE, mature ooctye extract. Note that that the antibody weakly recognizes a protein at the expected molecular weight of Kif2a at 85 kDa in CSF, but this is much stronger in the MOE, where phosphatases are inhibited by the addition of microcystin. Our interpretation is that only a small portion of Kif2a is phosphorylated in CSF, which lack centromeres. (C) Kinase assay using phosphoantibodies against pT70 shows that Aurora A in this case can phosphorylate Kif2a more efficiently than Aurora B/INCENPin box. This assay also shows that when these two kinases are mixed, the substrate is phosphorylated less than with Aurora A alone. (D) Immunofluorescence in Xenopus S3 cells shows localization of pT70 Kif2a to spindle poles during prometaphase and metaphase and to the spindle midzone during anaphase. Green, pT70; red, Ndc80 (kinetochores). Note that Kif2a localizes to centromeres in these cells (Supplemental Figure 8 in Knowlton et al., 2009), and therefore the absence of staining is not due to absence of Kif2a at these sites. (E) Sperm were added to Xenopus CSFs, and the resulting spindles were spun onto coverslips and stained for pT70. In extracts, pT70 Kif2a is localized to spindle poles and chromatin and enriched at centromeres. pT70 is dependent on Aurora activity, as this staining pattern is lost in the presence of Hesperadin. Red, pT70; blue, DNA; green, microtubules.