Abstract
The present study investigated the effects of the ethanolic extract (ESa), fractions, and compounds isolated from Sinningia aggregata in male Swiss mice on carrageenan-induced paw edema, neutrophil migration, mechanical hyperalgesia, formalin-induced nociception, and lipopolysaccharide-induced fever. The ESa did not alter edema, neutrophil migration, or fever at any of the doses tested. However, the ESa reduced phase II of formalin-induced nociception and carrageenan-induced mechanical hyperalgesia. The petroleum ether (PE) and ethyl acetate (EA) fractions and aggregatin D (AgD; isolated from the EA fraction) reduced formalin-induced nociception. Anthraquinones from the PE fraction were ineffective. AgD also inhibited carrageenan-induced mechanical hyperalgesia. Neither the ESa nor AgD altered thermal nociception or motor performance. Local administration of AgD also reduced hyperalgesia induced by carrageenan, bradykinin, tumor necrosis factor-α, interleukin-1β, cytokine-induced neutrophil chemoattractant, prostaglandin E2, and dopamine but not hyperalgesia induced by forskolin or dibutyryl cyclic adenosine monophosphate. The positive control dipyrone reduced the response induced by all of the stimuli. Additionally, glibenclamide abolished the analgesic effect of dipyrone but not the one induced by AgD. AgD did not change lipopolysaccharide-induced nitric oxide production by macrophages or the nociception induced by capsaicin, cinnamaldehyde, acidified saline, or menthol. These results suggest that the ESa has important antinociceptive activity, and this activity results at least partially from the presence of AgD. AgD reduced mechanical hyperalgesia induced by several inflammatory mediators through mechanisms that are different from classic analgesic drugs.
Introduction
Pain is one of the most pervasive problems in our society and has high social and economic impacts [1]. During inflammation, several mediators can activate and/or sensitize nociceptive fibers such as bradykinin (BK) [2], substance P (SP) [3], cytokines (e.g., tumor necrosis factor-α [TNF-α] and interleukin-1β [IL-1β]), prostaglandins and sympathetic amines [4]. In addition to pain, similar mediators are involved in edema formation and leukocyte infiltration [5–9]. If some mediators, particularly cytokines, reach the circulation, then they can cause fever by its actions in areas near the hypothalamus [10]. Several analgesics are used to treat a wide range of painful and inflammatory conditions including non-steroidal anti-inflammatory drugs (NSAIDs) [11], glucocorticoids [12] and opioids [13]. Aside from these drugs, other drugs have been used for specific painful conditions [14, 15]. Despite the great diversity of available antiinflammatory and analgesic drugs, their side effects and the ineffectiveness of some drugs in some conditions require the continuous search for new drugs.
The genus Sinningia belongs to the Gesneriaceae family and comprises 68 species that are distributed in South America. Many of them are found in Brazil [16]. The chemical composition of Sinningia species has been studied in the last few years. Flavonoids were isolated from S. cardinalis [17], and ethylcyclohexane derivatives and anthraquinones were identified in S. speciosa [18]. In S. allagophylla, lapachenol, 8-methoxylapachenol, anthraquinones, and naphthoquinones were found [19]. S. aggregata produces essential oil, anthraquinones, and aromatic compounds with a new skeleton named aggregatin A-D [20, 21].
Although the chemical composition of these plants is beginning to be known, few studies have investigated the pharmacological properties of the newly identified compounds. We recently found that an ethanolic extract from S. allagophylla exerted antinociceptive effects, an action related, at least partially, to the presence of 8-methoxylapachenol [22]. Anthraquinones were also found in these species, a class of compounds usually associated with antiinflammatory and antinociceptive activity in other species [23–25]. These observations prompted us to investigate the possible antinociceptive, antiinflammatory and antipyretic activity of the ethanolic extract obtained from the tuber of S. aggregata (ESa). Once the antinociceptive activity of the ESa was identified, we further investigated the activity of the fractions and isolated compounds obtained from the ESa. We identified antinociceptive effects in one of these compounds, aggregatin D (AgD), and evaluated its effectiveness against nociception induced by several mediators and ion channels agonists, and nitric oxide (NO) production to obtain some indications about its mechanism of action.
Materials and Methods
Animals
The experiments were conducted using male Swiss mice (25–35 g), that were housed 5 per cage containing sterile wood shavings under a 12h light/dark cycle, with controlled humidity (60–80%) and temperature (22 ± 1°C). Mice from the Biologycal Sciences Section standard breeding unit from the Federal University of Paraná were used and food and water were freely available. Animals were acclimatized to the experimental room at least 2 h before testing and were used only once throughout the experiments. The studies were performed in accordance with the current Brazilian (Conselho Nacional para o Controle de Experimentação Animal) and International guidelines for the care of laboratory animals. The animal procedures were approved by the Institutional Animal Care and Use Committee (Comissão de Ética para o Uso de Animais, Setor de Ciências Biológicas, Universidade Federal do Paraná CEUA/BIO-UFPR, authorization no. 628 and 722). The number of animals used was the minimum number necessary to show consistent effects of the drug treatments. All efforts were made to minimize animal suffering. At the end of the experiments, the animals were anesthetized with 60 mg/kg ketamine plus 7.5 mg/kg xylazine and euthanized by cervical dislocation.
Plant material
Sinningia aggregata (Ker-Gawl.) Whiehler (tubers) was collected in Tibagi, Paraná, Brazil, (24°30′32″ S, 50° 24′ 50″ W) in May 2007 and identified by Clarice B. Poliquesi. A voucher specimen (no. 290738) was deposited in the herbarium of Museu Botânico Municipal in Curitiba, Paraná, Brazil. S. aggregata is not an endangered species or protected, and authorization is not required for its collection. The authorization for accessing and studing samples from the Brazilian genetic heritage was obtained from the Brazilian governament through Conselho Nacional de Desenvolvimento Ciêntífico e Tecnológico (CNPq authorization no. 010087/2012-5).
Extraction and isolation
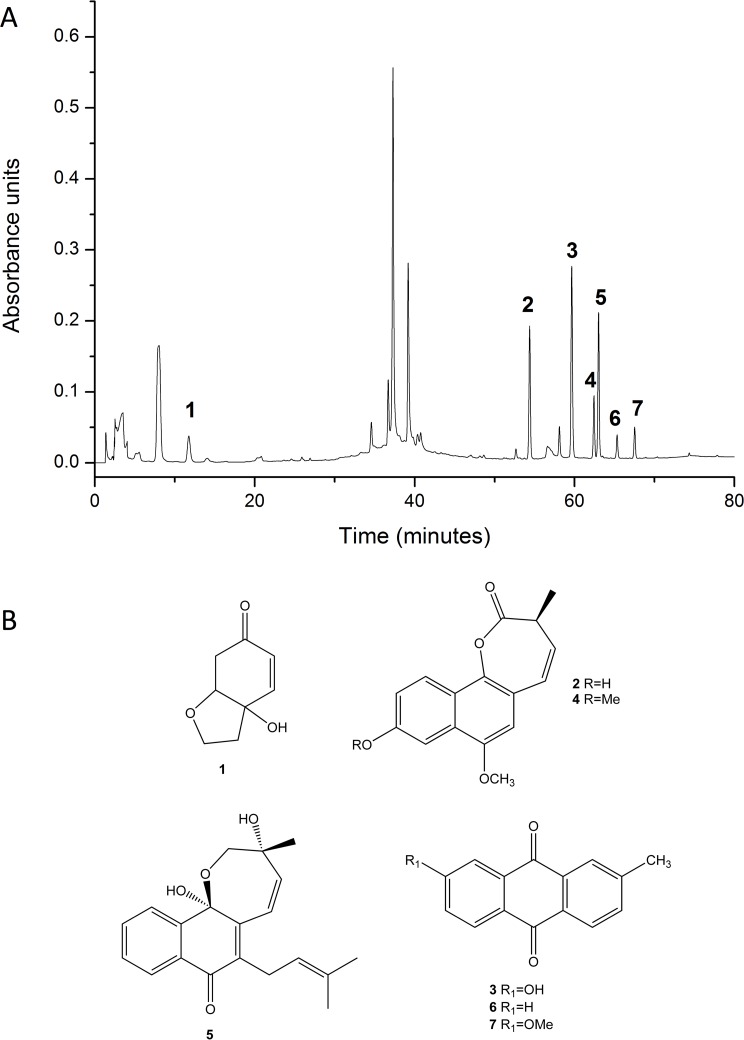
Dried and powdered tubers (50.5 g) were extracted at room temperature with ethanol (3 × 200 ml) and the solvent was removed under reduced pressure to yield the crude ESa (4.2 g). The crude ESa was suspended in ethanol:water 1:1 and subjected to partition with petroleum ether (PE), dichloromethane (DM), ethyl acetate (EA), and 1-butanol. After solvent removal the fractions yielded 4.1%, 5.0%, 23.3% and 35.1%, respectively. Biological assays showed that the activity was concentrated in the PE and EA fractions. After chromatographic fractioning, 2-methylanthraquinone (0.5%) and 7-methoxy-2-methylanthraquinone (0.5%) were isolated from the PE fraction and AgD (3%) was isolated from the EA fraction. The compounds were identified by 1D and 2D nuclear magnetic resonance and compared with data from the literature. A fingerprint of ESa together with the chemical structures of the identified compounds obtained by high-performance liquid chromatographyis is shown in Fig. 1, the detailed procedure for which was described previously [21].
Fig 1. Fingerprint of the ESa obtained from the tubers of S. aggregata.
The structures of the compounds identified are shown in panel B and are as follows: halleridone (1), aggregatin A (2), 7-hydroxy-2-methylanthraquinone (3), aggregatin C (4), aggregatin D (5), 2-methylanthraquinone (6) and 7-methoxy-2-methylanthraquinone (7).
Drugs
Carrageenan, lipopolysaccharide (LPS) E. coli 0111:B4, indomethacin, BK, prostaglandin E2 (PGE2), dopamine, forskolin, dibutyryl cyclic adenosine monophosphate (cAMP), glibenclamide, dipyrone, fentanyl, diazepam, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), ruthenium red, camphor, amiloride, capsaicin, cinnamaldehyde, and menthol were purchased from Sigma (St. Louis, MO, USA). Tumor necrosis factor- α (TNF-α), interleukin-1β (IL-1β) and cytokine-induced neutrophil chemoattractant 1 (CINC-1) were purchased from R&D Systems (Pittsburgh, PA, USA). Ketamine and xylazine were obtained from Syntec Laboratory (Cotia, SP, Brazil). Oxytetracycline hydrochloride was purchased from Pfizer (São Paulo, SP, Brazil). Dexamethasone was obtained from Química Santa Marina (Rio de Janeiro, RJ, Brazil).
Carrageenan-induced edema
Edema was measured as described previously [26]. Briefly, the mice were orally treated with ESa (10–100 mg/kg), vehicle (1% Tween 20), or dexamethasone (1 mg/kg, positive control). After 1 h, they received a 50 μl subcutaneous injection of carrageenan (300 μg into the right paw) suspended in sterile 0.9% saline. The contralateral paw received only saline and was used as a control. Edema was measured using a digital micrometer (Great, MT-045B, Shanghai, China) and is expressed as the difference between the paw thicknesses (in μm) 1 h before and 0.5, 1, 2, and 4 h after the carrageenan injection.
Lipopolysaccharide-induced fever
Abdominal body temperature was measured using data loggers (Subcue data loggers, Calgary, Canada) as described previously [26]. The data loggers were implanted intraperitoneally under 60 mg/kg ketamine plus 7.5 mg/kg xylazine anesthesia and aseptic conditions 1 week prior to the experiment. The animals were treated with 50 mg/kg oxytetracycline hydrochloride (i.m.) after surgery. Body temperature was continuously recorded at 15-min intervals from 2 h before until 6 h after an intraperitoneal injection of 100 μg/kg LPS. Controls were treated with the same volume of pyrogen-free saline (vehicle). The ESa (30 mg/kg) or vehicle (1% Tween 20) was administered orally 1 h before the LPS injection. During the experiment, the room temperature was kept at 30 ±1°C (i.e. a thermoneutral zone for mice) [27].
Carrageenan-induced neutrophil migration
To investigate the effect of the ESa on neutrophil migration, the animals were treated with the ESa (10–100 mg/kg, p.o.), vehicle (1% Tween 20), or dexamethasone (1 mg/kg, positive control). One hour later, they received carrageenan (300 μg/paw) or saline. The animals were euthanized, as described above, 4 h after the carrageenan injection, and the tissues of the injected paws were removed. Samples were processed, and myeloperoxidase activity was assessed as described previously [28]. Protein content in the samples was measured using Bradford’s method. Enzymatic activity was determined by measuring the optical density (OD) at 630 nm and is expressed as OD per mg of protein.
Formalin-induced nociception
The procedure was similar to the one described previously [29]. Briefly, the animals were placed in glass cylinders (20 cm diameter) for adaptation for 30 min. The mice were then orally treated with the ESa (10–100 mg/kg), PE fraction (1.30 mg/kg), DM fraction (1.50 mg/kg), EA fractoins (7.00 mg/kg), or the isolated compounds AgD (0.21 mg/kg), methylanthraquinone (1.25 mg/kg), and 7-methoxy-2-methylanthraquinone (1.25 mg/kg). The control animals received the same volume of vehicle (10 ml/kg, 1% Tween 20, p.o.) or indomethacin (5 mg/kg, p.o., positive control). After 1 h, the animals received an injection of 20 μl of 2.5% formalin (0.92% formaldehyde) in phosphate-buffered saline (PBS) in the right hindpaw. The time that the animals spent licking and elevating the injected paw (i.e. nociceptive behavior) was recorded in 5-min blocks for 30 min. The first 5-min block was considered as phase I of the test (i.e. the neurogenic phase) and the 15–30 min period was considered phase II (i.e. the inflammatory phase).
Carrageenan-induced mechanical hyperalgesia
The mechanical threshold was measured by using von Frey filaments (Stoeling, Chicago, IL, USA) in the up-and-down paradigm as described previously [30, 31]. The mice were first acclimated (1 h) in individual clear Plexiglas boxes (9 x 7 x 11 cm) on an elevated wire mesh platform to allow access to the plantar surface of the hindpaws. The paw was then touched with a series of eight von Frey fileaments with logarithmic increments of force (0.008, 0.02, 0.07, 0.16, 0.4, 1.0, 2.0, and 4.0 g). The von Frey filaments were applied perpendicularly to the plantar surface with sufficient force to cause slight buckling against the paw and held in place for approximately 2–4 s. The absence of paw lifting after this time led to the use of the next filament with increased force. Paw lifting indicated a positive response and led to the use of the next weaker filament. This paradigm continued until six measurements were collected. The 50% mechanical paw withdrawal threshold was then calculated from these scores as described previously [31]. The animals were then treated with the ESa (3–30 mg/kg), the isolated compound AgD (0.21 mg/kg), or vehicle (1% Tween 20) orally or AgD (0.07–7 ng into the right or left paw) or the respective vehicle (0.1% Tween 20 in sterile saline, 20 μl) applied locally). Sixty minutes after oral treatment or 15 min after local treatment, the animals received a 20 μl injection of carrageenan (300 μg/paw) into the right hindpaw. The withdrawal response was measured again 3 h after the carrageenan injection.
In mice, the hyperalgesia induced by carrageenan induces the release of TNF-α and keratinocyte-derived chemokine (KC) which stimulate the release of IL-1β [4]. IL-1β and KC induce the release of prostaglandins but KC also induces the release of sympathetic amines. Both prostaglandins and sympathetic amines induce the synthesis of cAMP [4]. BK evokes nociception through a separate pathway [2]. To evaluate whether AgD affects any particular point of this cascade, animals were treated with AgD (7 ng/paw) or the positive control dipyrone (320 μg/paw) or vehicle (0.1% Tween 20). After 15 min they received local injections of different nociceptive stimuli: TNF-α (1 pg/paw), IL-1β (0.5 pg/paw), CINC-1 (10 pg/paw, which activates the same receptor as KC), PGE2 (100 ng/paw), BK (500 ng/paw), dopamine (3 μg/paw), dibutyryl cAMP (dbcAMP, 5 μg/paw), and forskolin (1 μg/paw). Mechanical hyperalgesia was measured after 3 h.
To evaluate if AgD exerts its action by activating NO/cyclic guanosine monophosphate (cGMP)/K+ pathway similarly to dipyrone, the animals were treated with vehicle (1% Tween 80 in sterile saline) or the K+ channel blocker glibenclamide (80 μg/paw). After 30 min they received AgD (7 ng/paw), the positive control dipyrone (320 μg/paw), or vehicle (0.1% Tween 20). After 15 min, the animals received a local injection of PGE2 (100 ng/paw). Dipyrone was included as a positive control in this series of experiments because it has been shown to reduce hyperalgesia induced by of all of these stimuli. The doses of nociceptive stimuli, dipyrone, and glibenclamide were based on previous studies [2, 4, 32–35].
Hot-plate test
The hot-plate test was performed as described previously [26]. The temperature of the hot plate was maintained at 55 ± 1°C. Basal latency was measured, and only animals that presented a basal latency between 7–15 s were used. After 30 min, the mice were orally treated with the ESa (10, 30 or 100 mg/kg), AgD (0.21 or 2.1 mg/kg), vehicle (1% Tween 20) or with fentanyl (0.5 mg/kg, s.c., positive control). One hour after treatment with the extract, compound, and vehicle, or 15 min after treatment with fentanyl, the postdrug latency was evaluated. The cut-off time was 30 s. Hot-plate latencies were then converted to a percentage of the maximal possible effect (%MPE): %MPE = (postdrug latency—basal latency) / (cut-off time—basal latency) x 100.
Locomotor performance
To evaluate the possible nonspecific muscle-relaxant or sedative effects of the ESa and AgD, the mice were subjected to the rotarod test (Ugo Basile, Model 7600, Varese, Italy) [26]. The animals were treated with ESa (10, 30, or 100 mg/kg), AgD (0.21 or 2.1 mg/kg), vehicle (1% Tween 20) or diazepam (5.0 mg/kg, s.c., positive control). One hour after ESa or AgD treatment or 15 min after diazepam treatment, animals were submitted to the rotarod test. The cut-off time was set at 180 s.
Nitric oxide production by peritoneal macrophages
To evaluate wether AgD acts directly on macrophages, cells from the peritoneal cavity were obtained as described previously [36]. Briefly, the peritoneal cavity of halothane-anesthetized mice was washed with 6 ml of sterile PBS that contained 5U/ml heparin. The fluid was then centrifuged at 1000 x g, at 4°C for 10 min to separate the cells. The cells were then resuspended in RPMI 1640, pH 7.4 and washed twice. The cells (106 cells/well) were then placed in a 96-well plate and left to adhere 1 h at 37°C in a 5% CO2 atmosphere. Non-adherent cells were washed away with RPMI 1640. The adhered cells were exposed to RPMI 1640 medium that contained AgD (4, 40, 400, and 4000 ng/ml) with or without LPS (100 ng/ml). Control monolayers were exposed only to RPMI 1640 or dexamethasone (9 μg/ml) plus LPS. Samples were collected after 4 h and kept at −20°C for NO evaluation using the Griess reaction as described previously [37]. For viability evaluation, the cells were incubated with 100 μl MTT (5 mg/ml) in RPMI 1640 medium for 24 h at 37°C in a 5% CO2 atmosphere. After this period, the formazan precipitate was dissolved with 10% sodium dodecyl sulfate solution that contained 1 M HCl. Absorbance was read at 550 nm after 4 h [38, 39].
Nociception induced by TRP channel agonists
To test wether transient receptor potential V1 (TRPV1), TRPA1, TRPM8 and acid-sensing ion channel [40] channels might constitute potential specific targets for the antinociceptive actions of AgD, we tested the effects of AgD against nociceptive responses elicited by activators of each channel. Ten minutes following local treatment with AgD (7 ng/paw) or the positive controls ruthenium red (nonselective TRP antagonist, 2 nmol/paw), camphor (TRPA1 antagonist, 250 ng/paw), or amiloride (epithelial Na+ channel blocker, 3 μg/paw), the mice received a 20 μL intraplantar injection of the TRPV1 agonist capsaicin (0.1 nmol/paw), TRPA1 agonist cinnamaldehyde (10 nmol/paw), ASIC agonist acidified saline (2% acetic acid in 0.9% saline, pH 1.98, 20 μl/paw), or TRPM8 agonist menthol (2.4 μmol) into the ventral surface of the right paw. Each animal was then placed, immediately and alone, into a glass cylinder (20 cm diameter) that was positioned on a platform in front of a mirror to enable full view of the hindpaws. The amount of time that the animal spent licking the injected paw was recorded with a chronometer over 5 min (for capsaicin or cinnamaldehyde) or 20 min (acidified saline and menthol) and used as an index of nociceptive behavior intensity. The doses were based on previous studies [41, 42]
Statistical analysis
The results are presented as mean ± s.e.mean. Statistical significance among groups was assessed using two-way repeated measures (oedema and fever) analysis of variance (ANOVA) or one-way ANOVA for the subsequent experiments. Significant mean effect and interactions in the ANOVAs were followed by the Bonferroni post hoc test. Dose-response curves in the mechanical hyperalgesia experiments were analyzed by one-way ANOVA followed by Fisher’s Least Significant Difference post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Effects of the ESa on carrageenan-induced edema, neutrophil migration, and LPS-induced fever
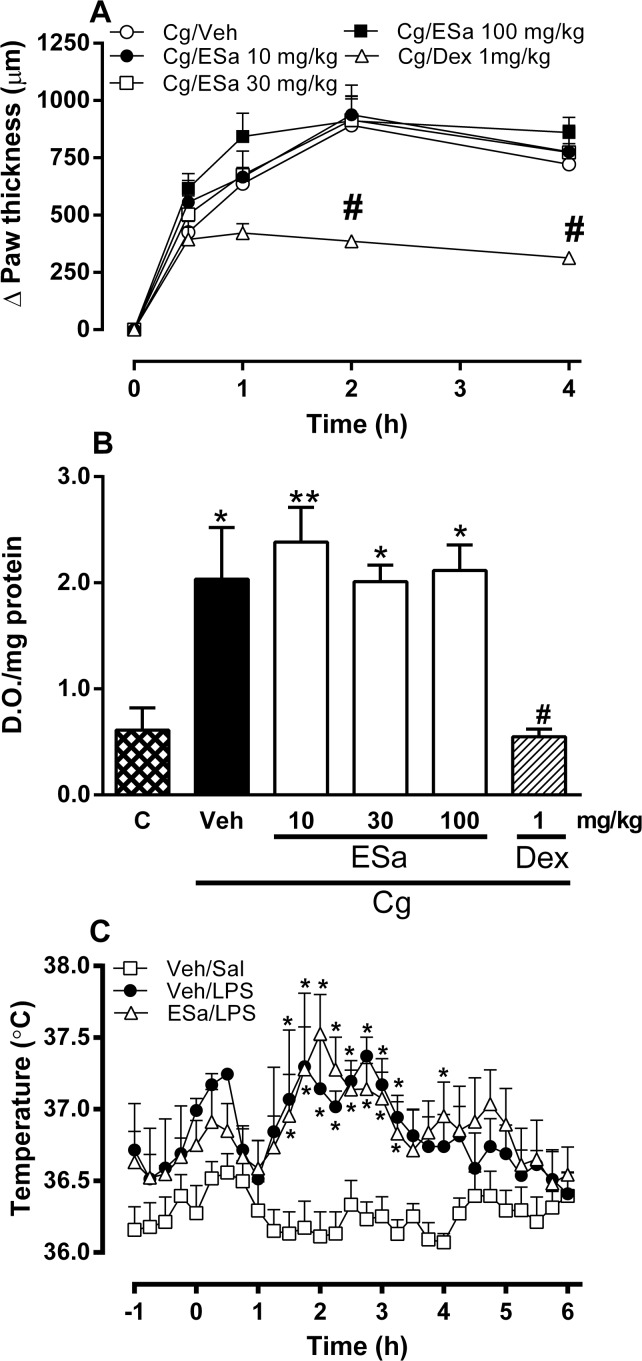
Carrageenan injection in the paw induced an edema response that began at 30 min and peaked at 2 h (Fig. 2A). Carrageenan also increased myeloperoxidase activity (an indicator of neutrophil migration) in the paw after 4 h (Fig. 2B). Oral treatment with the ESa (10–100 mg/kg) did not inhibit carrageenan-induced paw edema or myeloperoxidase activity whereas dexamethasone treatment significantly reduced both (Fig. 2A and B, respectively). The intraperitoneal injection of LPS induced a febrile response that began 1.5–2 h after the injection and persisted until 4–4.5 h. Oral treatment of the animals with the Esa (30 mg/kg) did not change LPS-induced fever (Fig. 2C).
Fig 2. Effect of ESa on carrageenan-induced edema and neutrophil migration and on lipopolysaccharide-induced fever.
Animals were treated with ethanolic extract form S. aggregata (ESa, 10 to 100 mg/kg, as indicated), Dexamethasone (Dex, 1 mg/kg), or the appropriate vehicles (Veh) by oral route. One hour after the oral treatment animals were injected with carrageenan (Cg, 300 μg) in the paw (panels A and B) or LPS (100 μg/kg, i.p, panel C). On panel B, the control (C) animals are non-injected animals. Data show the mean ± s.e.mean of the change in the paw thickness (μm, panel A), optical density (D.O./mg protein, panel B) or body temperature (°C, panel C) (n = 7–14). Symbols denote statistical difference in relation to the control (C) or Veh/Sal group (* P<0.05, **P<0.01) or to Veh/Cg (#p<0.05).
Effects of the ESa on formalin-induced nociception and carrageenan-induced mechanical hyperalgesia
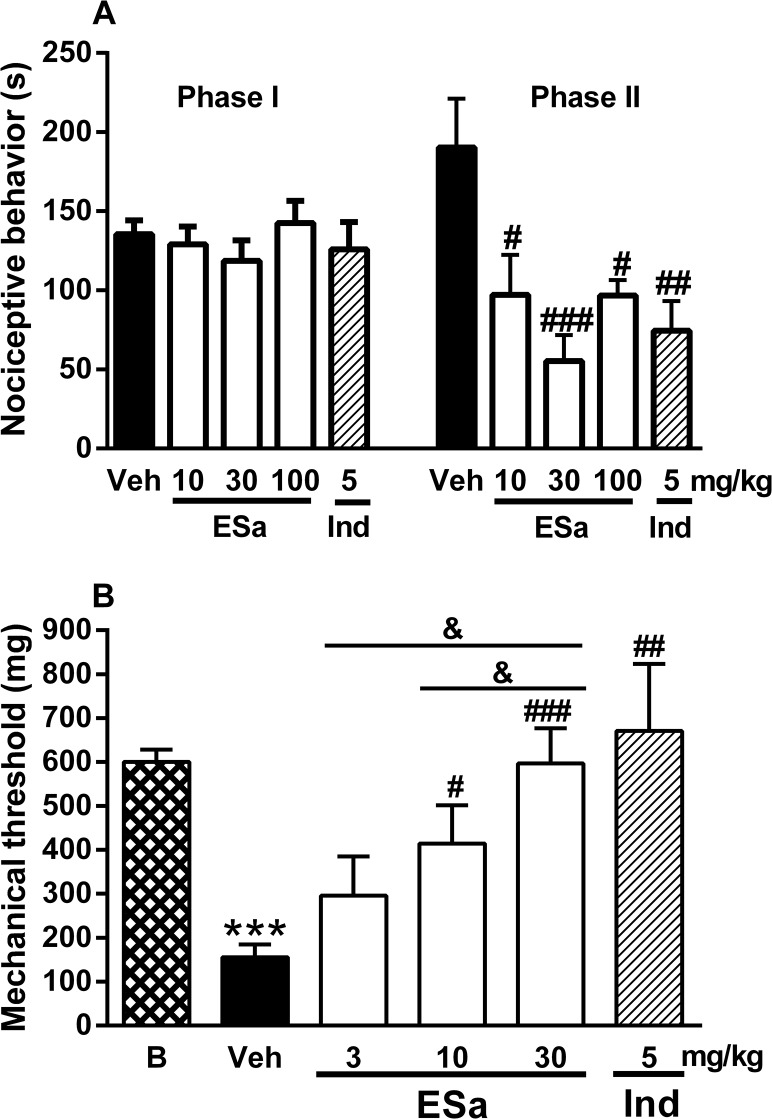
Formalin injection induced nociceptive behavior during phase I (approximately 136 s) and on phase II (approximately 190 s; Fig. 3A). ESa (10–100 mg/Kg) significantly inhibited the inflammatory phase (phase II) of formalin-induced licking, with a maximal reduction of 69 ± 10% whereas no inhibition was seen in the neurogenic phase (phase I). These results were similar to those obtained with indomethacin, which was ineffective in phase I but inhibited 52 ± 9% of phase II (Fig. 3A).
Fig 3. Effect of ESa on nociception.
Animals were treated with ethanolic extract form S. aggregata (ESa, 10 to 100 mg/kg, as indicated), Indomethacin (Ind, 5 mg/kg) or Diazepam (Dzp, 5 mg/kg) by oral route or with Fentanil (Fent, 0.5 mg/kg, s.c.) or the appropriate vehicles (Veh) as described in Methods. One hour after the oral treatment or 15 min after s.c. tretatment, animals were injected with formalin 2.5% in the paw (panel A) or carrageenan (300 μg) in the paw (panels B) or submitted to the hot plate (panel C) or rota-rod task (panel D). On panel B, basal (B) mechanical threshold means the threshold before any injection. Data show the mean ± s.e.mean of the change in the nociceptive behavior (s, panel A), mechanical threshold (mg, panel B), MEP (%, panel C) and motor performance (s, panel D) (n = 7–14). Symbols denote statistical difference in relation to the basal (B) group (***P<0.001) or to Veh-treated animals (# P<0.05, ## P<0.01 and ### P<0.001).
A maximal effect was already observed with 30 mg/kg ESa in the formalin test. Therefore, this dose was the higher dose chosen for the following experiment. The injection of carrageenan in the hindpaw significantly reduced the mechanical threshold 3 h after the injection (Fig. 3B). Oral treatment with ESa (3–30 mg/kg) dose-dependently inhibited carrageenan-induced mechanical hyperalgesia (Fig. 3B). Indomethacin exerted a similar effect on mechanical hyperalgesia.
Effects of the ESa in the hot-plate test and motor performance
Oral injection of the ESa 10–100 mg/kg did not change the latency time in the hot-plate test at 55°C compared with vehicle-treated animals whereas fentanyl significantly increased the %MPE compared with vehicle-treated animals (S1 Fig.). Similarly, oral administration of the ESa (10 to 100 mg/kg) did not significantly affect motor behavior in the rotarod test compared with animals that received only the vehicle (S1 Fig.). Conversely, the positive control diazepam significantly reduced motor coordination (79 ± 9%, S1 Fig.).
Effects of ESa fractions on formalin-induced nociception
Oral treatment with PE and EA fractions at doses of 1.3 mg/kg and 7 mg/Kg, respectively, but not the DM fraction at a dose of 1.5 mg/kg (doses based on yield) obtained from the ESa, significantly inhibited phase II of formalin-induced nociceptive behavior with maximal inhibition of 57 ± 7% for PE and 43 ± 8% for EA with no changes observed in phase I (S2 Fig.). Similar results were obtained for the positive control indomethacin (55 ± 9% reduction in phase II, S2 Fig.). The doses of the fractions were based on the yield considering an effective dose of 30 mg/kg ESa.
Effect of the isolated compounds on formalin-induced nociception and on carrageenan-induced mechanical hyperalgesia
Treatment with AgD (0.21 mg/kg, p.o.), a compound isolated from the EA fraction, significantly inhibited the inflammatory phase of formalin-induced licking with maximal inhibition of 56 ± 7% but no significant changes were observed in phase I (Fig. 4A). Treatment with 2-methylanthraquinone and 7-metoxy-2-methylanthraquinone obtained from the PE fraction did not alter the nociceptive behavior induced by formalin (data not shown).
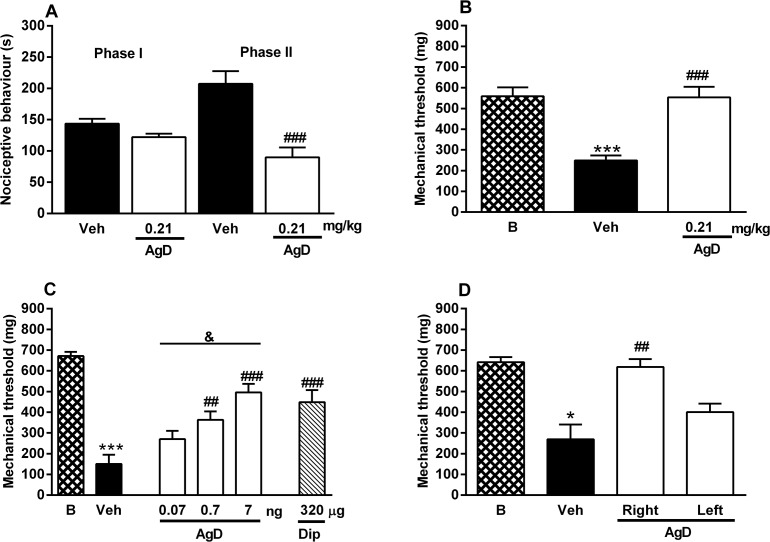
Fig 4. Effect of AgD on nociceptive behavior induced by formalin and on mechanical hyperalgesia induced by carrageenan.
Animals were treated with Aggregatin D (AgD) 0.21 mg/kg or vehicle (Veh), by oral route 1 h before the administration of formalin (2.5%, panel A) or carrageenan (Cg, 300 μg, panel B) into the right paw or AgD (0.07, 0.7, 7 ng/paw), Dipyrone (Dip, 320 μg/paw) or vehicle (Veh) 15 min before the injection of Cg (panel C) into the right paw. On panel D, AgD (7ng/paw) was injected in right or left paw as indicated and Cg (300 μg) was injected in the right paw. Basal (B) threshold was evaluated before any injection in panels B, C and D. Formalin-induced nociceptive behavior was evaluated in phase I (0–5 min) or in phase II (15 to 30 min) and the mechanical threshold was evaluated again 3 h after the injection of Cg. Bars represent the mean±s.e.mean of the nociceptive behavior (s) induced by formalin in each phase or the mechanical threshold (n = 10–20). Symbols denote statistical difference in relation to basal threshold (*P<0.05, ***P<0.001) or to veh-treated group (## P<0.01, ### P<0.001).
The injection of carrageenan in the hindpaw significantly reduced the mechanical threshold after 3 h (Fig. 4B). Oral treatment with AgD (0.21 mg/kg, dose based on the yield) abolished the carrageenan-induced mechanical hyperalgesia (Fig. 4B).
Local treatment with AgD dose-dependently reduced the carrageenan-induced mechanical hyperalgesia (Fig. 4C) 3 h after the carrageenan injection. AgD (0.7 and 7 ng) significantly reduced mechanical hyperalgesia (41% and 66% inhibiton, respectively), whereas a lower dose (0.07 ng) had no effect. Dipyrone (320 μg) was used as a positive control and had similar effects (57% inhibition, Fig. 4C). Additionally, AgD treatment in the paw contralateral to the carrageenan had no significant effect on mechanical hyperalgesia in contrast to the total inhibition observed with AgD administration in the same paw as the carrageenan (Fig. 4D). The 7 ng dose of AgD was more effective, and this dose was chosen for the subsequent experiments.
Effect of AgD in the hot plate test and motor performance
Oral administration of AgD (at the same doses that inhibited nociception or a 10-times higher dose) did not change the latency in the hot-plate test at 55°C compared with vehicle-treated animals whereas fentanyl significantly increased the %MPE compared with vehicle-treated animals (S1 Fig.). Similarly, administration of AgD (at the same doses and time points that inhibited nociception and a 10-times higher dose) did not significantly affect motor performance of the animals tested in the rotarod test compared with animals that received only the vehicle (S1 Fig.).
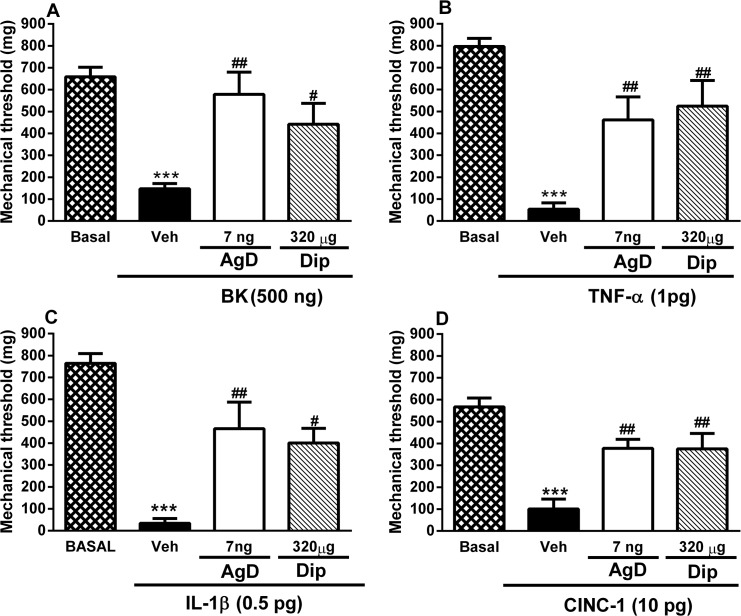
Effect of AgD on BK-, TNF-α-, IL-1β-, and CINC-1-induced mechanical hyperalgesia
The injection of BK, TNF-α, IL-1β and CINC-1 in the paw significantly reduced the mechanical threshold (Fig. 5A-5D). Local treatment with AgD (7 ng/paw) reversed mechanical hyperalgesia induced by BK and the cytokines by 84%, 55%, 50%, and 59%, respectively. Dipyrone was used as a positive control and also inhibited mechanical hyperalgesia induced by BK and the cytokines by 57%, 63%, 50%, and 59% respectively.
Fig 5. Effect of AgD on mechanical hyperalgesia induced by BK and cytokines.
Animals were treated with Aggregatin D (AgD, 7 ng/paw), Dipyrone (Dip, 320 μg/paw) or vehicle (Veh) 15 min before the injection of bradykinin (BK, 500 ng/paw, panel A), tumor necrosis factor-α (TNF-α, 1 pg/paw, panel B), interleukin-1β (IL-1β, 0.5 pg/paw, panel C) or cytokine-induced neutrophil chemoattractant (CINC-1, 10 pg/paw, panel D) in the right paw. Basal (B) threshold was evaluated before any injection. The mechanical threshold was evaluated again 3 h after the injection of the nociceptive stimuli. Bars represent the mean±s.e.mean of the mechanical threshold (mg, n = 8–10). Symbols denote statistical difference in relation to basal threshold (***P<0.001) or to veh-treated group (# P<0.05, ## P<0.01).
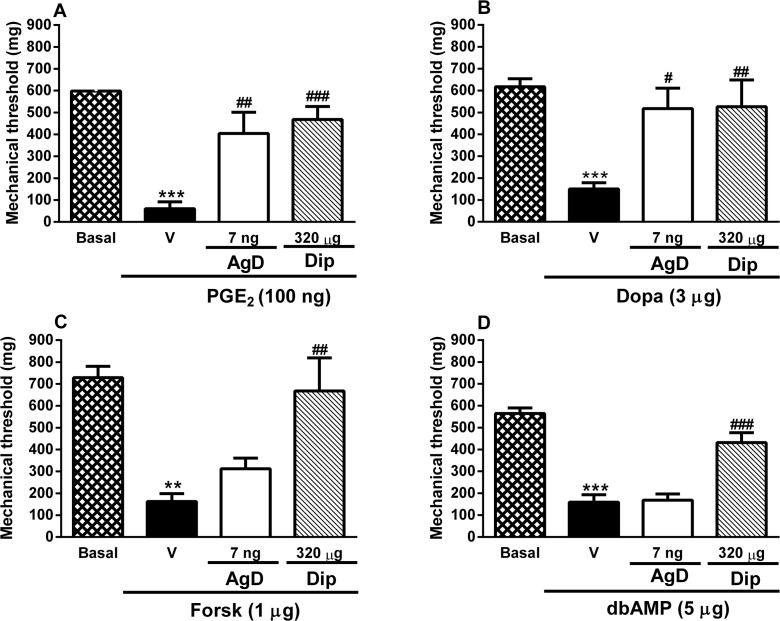
Effect of AgD on PGE2-, dopamine-, forskolin-, and dbcAMP- induced mechanical hyperalgesia
The injection of PGE2, dopamine, forskolin, and dbcAMP in the paw significantly reduced the mechanical threshold (Fig. 6A-6D). Local treatment with AgD (7 ng/paw) reduced the mechanical hyperalgesia induced by PGE2 and dopamine by 64% and 78%, respectively (Fig. 6A and 6B). AgD did not alter the reduction of the mechanical threshold induced by forskolin or dbcAMP (Fig. 6C and 6D). Dipyrone was used as a positive control and reduced the mechanical hyperalgesia induced by PGE2, dopamine, forskolin and dbcAMP by approximately 76%, 80%, 89%, and 67%, respectively.
Fig 6. Effect of AgD on mechanical hyperalgesia induced by PGE2, dopamine, forskolin and dbAMPc.
Animals were treated with Aggregatin D (AgD, 7 ng/paw), Dipyrone (Dip, 320 μg/paw) or vehicle (Veh) 15 min before the injection of prostaglandin E2 (PGE2, 100 ng/paw, panel A), Dopamine (Dopa, 3 μg/paw, panel B), forskolin (Forsk, 1 μg/paw, panel C) or dybutiryl cAMP (dbcAMP, 5 μg/paw, panel D) in the right paw. Basal (B) threshold was evaluated before any injection. The mechanical threshold was evaluated again 3 h after the injection of the nociceptive stimuli. Bars represent the mean±s.e.mean of the mechanical threshold (n = 6–16). Symbols denote statistical difference in relation to basal threshold (**P<0.01, ***p<0.001) or to Veh-treated group (## P<0.01, ### P<0.001).
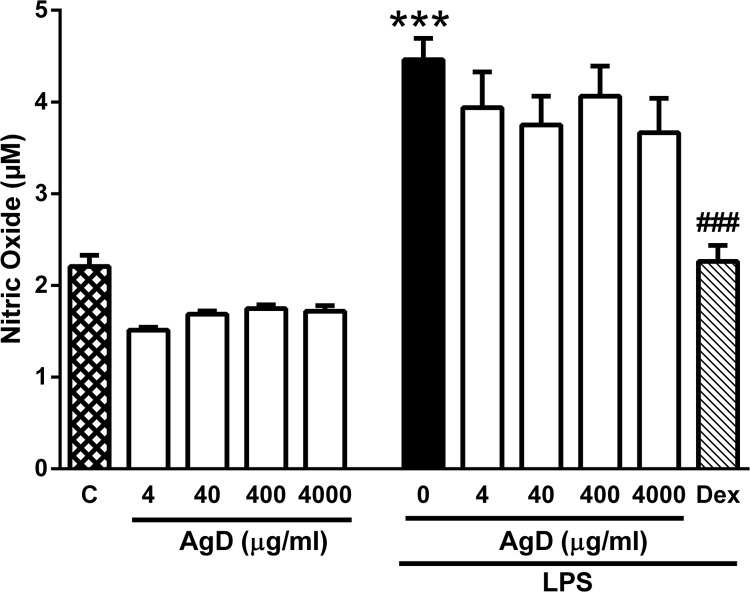
Effect of AgD on NO release
The basal production of NO (measured as nitrite/nitrate) by peritoneal macrophages was not significantly changed by treating the cells with AgD, although a small reduction of the NO concentration was observed with all concentrations tested (Fig. 7). LPS significantly increased NO production, but treatment of the cells with AgD did not modify this production (Fig. 7). Conversely, treatment of the cells with the glucocorticoid dexamethasone abolished the increase in NO production induced by LPS (Fig. 7). None of the treatments significantly altered cell viability which was always higher than 95% (data not shown).
Fig 7. Effect of AgD on NO release by peritoneal macrophages.
Mouse peritoneal macrophages (106 cell/well) were treated with Aggregatin D (AgD, 4–4000 ng/ml) or with RPMI 1640 (control, C) in the presence or absence of lipopolysaccharide (LPS, 100 ng/ml). Positive control cells were treated with Dexamethasone (Dex, 9 μg/ml) in the presence of LPS. NO production was quantified through the Nitrite/nitrate production by the Griess method. Bars show the mean±s.e.mean of the nitrite/nitrate (μM) in quadruplicates. Symbols denote statistical difference in relation to control (RPMI) group (***P<0.001) or to LPS-treated group (### P<0.001).
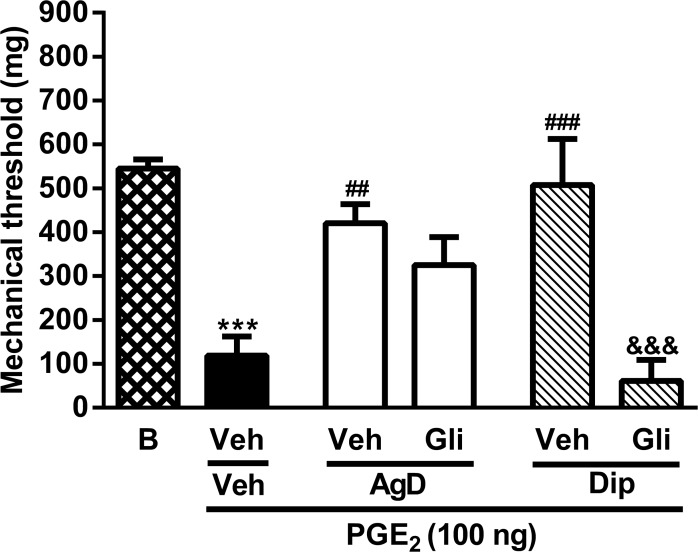
Effect of glibenclamide on the antinociceptive effect of AgD
The injection of PGE2 reduced the mechanical threshold of the paw by approximately 78% (Fig. 8). Treatment with both AgD and dipyrone completely reversed the mechanical hyperalgesia induced by PGE2. Administration of the potassium channel blocker glibenclamide prevented the antinociceptive effect of dipyrone (positive control) but not of AgD (Fig. 8).
Fig 8. Effect of the potassium channel blocker glibenclamide on the anti-hyperalgesic effect of AgD.
Animals were treated with the potassium channel blocker glibenclamide (Gli, 80 μg/paw) or the same volume of vehicle (Veh, Tween 80 1% in saline). After 30 min, animals received Aggregatin D (AgD, 7 ng/paw), Dipyrone (Dip, 320 μg/paw) or vehicle (Veh) followed by an injection of of prostaglandin E2 (PGE2, 100 ng/paw) 15 min later. Basal (B) threshold was evaluated before any injection. The mechanical threshold was evaluated again 3 h after the injection of the nociceptive stimuli. Bars represent the mean±s.e.mean of the mechanical threshold (n = 5–10). Symbols denote statistical difference in relation to basal threshold (***P<0.001), to Veh/Veh-treated group (## P<0.01, ### P<0.001) or to the Veh/Dip-treated group (&&& P<0.001).
Effect of AgD on nociception induced by capsaicin, cinnamaldehyde, acidic saline and menthol
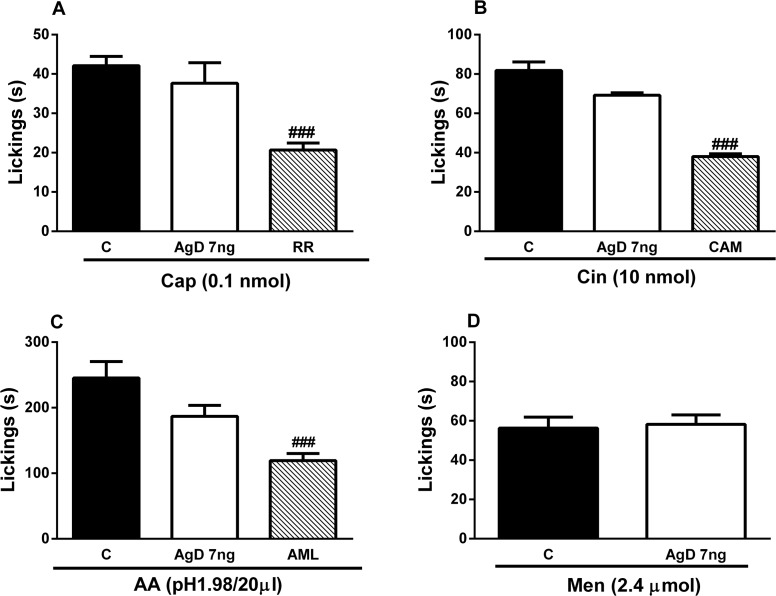
Capsaicin, cinnamaldehyde, acidic saline and menthol were injected in the hindpaw of the animals inducing a clear nociceptive behavior (Fig. 9) compared with saline-treated animals which did not show any response. Preatrement with these channels blockers ruthenium red, camphor and amiloride significantly decreased the nociceptive behavior induced by these compounds (Fig. 9A-9C). AgD had no effect on nociceptive behavior induced by capsaicin, cinnamaldehyde, menthol, and acidic saline, which are activators of TRPV1, TRPA1, TRPM8, and ASIC3 channels (Fig. 9).
Fig 9. Effect of AgD on nociceptive behavior induced by capsaicin, cinnamaldehyde, acidified saline and menthol.
Animals were treated with AgD (7 ng/paw), ruthenium red (RR 1.5 μg/paw), camphor (CAM, 250 ng/paw) or amiloride (AML, 3 μg/paw) or vehicle (Veh) 10 min before the injection of capsaicin (0.1 nmol/paw, panel A), cinnamaldehyde (10 nmol/paw, panel B), acidified saline (pH1.98, 20 μl/paw, panel C) or menthol (2.4 μmol/paw, panel D) in the right paw. The nociceptive behavior was evaluated during 5 min in panels A and B and for 20 min in panels C and D. Bars represent the mean±s.e.mean of the nociceptive behavior (s) (n = 5–8 animals). Symbols denote statistical difference in relation to Veh-treated group (### P<0.001).
Discussion
The present study showed that the ethanolic extract obtained from the tubers of S. aggregata has important antinociceptive activity, in which it blocked the inflammatory phase of overt nociception induced by formalin and mechanical hyperalgesia induced by carrageenan. However, the ESa did not exert an antiinflammatory or antipyretic effect. A new compound identified in this plant, AgD, shared this antinociceptive activity, likely acting at peripheral sites. Additionally, AgD blocked mechanical hyperalgesia induced by BK, TNF-α, IL-1β, CINC-1, PGE2 and dopamine but not forskolin, dbcAMP, capsaicin, cinnamaldehyde, menthol, or acidic saline. The analgesic effect of AgD also did not appear to involve the NO/cGMP/K+ channel pathway.
The ESa was effective against inflammatory nociception (formalin-induced and mechanical hyperalgesia). However, ESa, at least at the doses tested, does not act similarly to NSAIDs or glucocorticoids, because these drugs effectively inhibit edema formation [43], neutrophil migration [44] and fever [26, 45]. The ESa also did not appear to act similarly to opiods, another class of commonly used analgesic drugs, because opioids effectively block the first phase of formalin-induced nociceptive behavior in mice [29] and physiological heat-induced nociception (S1 Fig.). Although dipyrone and acetaminophen are two NSAIDs that are known to have weak anti-inflammatory action [46, 47] they exert antipyretic effects at similar or lower doses than those required for their analgesic effects [48, 49]. Therefore, these data suggest that the ESa contain compounds that mainly have antinociceptive effects that act specifically on nociceptor sensitization.
After fractioning, two of these ESa-derived fractions showed a similar antinociceptive activity: the PE fraction and EA fraction. From the PE fraction two anthraquinones, 2-methylanthraquinone and 7-methoxy-2-methylanthraquinone, were isolated. Anthraquinones are potential antinociceptive drugs. For example, the anthraquinones diacerhein and emodin possess antinociceptive activity in different models [50, 51]. Nevertheless, both anthraquinones that were isolated from the PE fraction failed to change formalin-induced nociception at the doses tested. Higher doses were not tested but these anthraquinones are unlikely responsible for the antinociceptive activity of the ESa because the doses used were the same as the PE fraction. Sitosterol, a compound found in the PE fraction, possesses antinociceptive activity at doses that are 10 times higher than the one used in the present study [52], suggesting that it is not responsible for the activity of this fraction. Therefore, compounds other than those isolated in the present study may be responsible for the antinociceptive activity of the PE fraction. This will be an issue for future studies.
Conversely, a new hydronaphthoquinone derivative identified in the EA fraction, named AgD, very effectively reduced both carrageenan-induced mechanical hyperalgesia and the formalin-induced nociception. These data suggest that the antinociceptive activity of the ESa is at least partially related to the presence of AgD. Notably, the doses of the ESa and AgD that completely abolished carrageenan-induced hyperalgesia only partially reduced the nociceptive behavior induced by formalin. This may be attributable to the fact that formalin evokes very complex behavior that involves the sensitization and/or activation of nociceptors, and AgD specifically reduces nociceptor sensitization. Additionally, the ESa and AgD failed to produce antinociceptive effects when a thermal stimulus (i.e., the hot plate) was used thus substantiating their preferential peripheral action in nociceptive responses of inflammatory origin. We also evaluated the mice in the rota rod test, and confirmed that neither the ESa nor AgD affected motor performance at the doses tested which could otherwise affect the results of the nociception tests. AgD did not affect the motor performance even at a dose that was 10 times higher than the antinociceptive dose.
AgD also effectively reduced mechanical hyperalgesia when administered locally, suggesting that it acts directly at the inflammatory site. The local effect was confirmed when the same dose of AgD that was injected in the contralateral paw exerted no antinociceptive effect. Carrageenan-induced mechanical hyperalgesia in mice depends on the release of BK, TNF-α, IL-1β, and KC and subsequently of sympathetic amines and prostaglandins [53–55]. Based on this sequence of mediators, we attempted to unravel the mechanism of action of AgD. Our results clearly showed that AgD prevented sensitization induced by BK, TNF-α, IL-1β, and CINC-1, a chemokine that shares the same receptor with KC [56]. These results suggest that AgD was not blocking the synthesis or release of these mediators and acted after their release at the inflammatory site.
However, AgD also significantly reduced mechanical hyperalgesia induced by both PGE2 and dopamine, which are described as the final mediators of inflammatory hyperalgesia [4, 53, 54]. PGE2 biosynthesis depends on the conversion of membrane arachidonic acid by the action of cyclooxygenase [11]. Therefore, the blockade of PGE2-induced hyperalgesia suggests that AgD does not act as an NSAID by inhibiting cyclooxygenase-2 activity. Once released, PGE2 interacts with G protein-coupled receptors subtypes EP2 and/or EP4, which are expressed in peripheral sensory neurons. The activation of these receptors results in the activation of adenylyl cyclase and increase in the levels of cAMP, which directly promotes the activation of protein kinase A. Protein kinase A, in turn, has actions on various ion channels that sensitize nociceptors [57, 58]. Alternatively, sympathetic amines have been shown to be involved in the development of hyperalgesia by functionally upregulating nociceptors [59, 60]. Dopamine is a sympathetic amine that is released during inflammation and promotes the direct sensitization of nociceptive neurons in a manner that depends on the activation of dopamine D1 receptors that stimulate the adenylyl cyclase/cAMP pathway [61–64].
Therefore our next step was to evaluate wether AgD reverses mechanical hyperalgesia by acting on these intracellular components. Mechanical hyperalgesia was induced by the adenylyl cyclase activator forskolin and dbcAMP, a permeable analogue of cAMP which is a direct activator of protein kinase A. Both, forskolin and dbcAMP reduced the paw withdrawal threshold to the same extent as PGE2 and dopamine. However, at the dose tested, AgD did not reverse mechanical hyperalgesia induced by these agents. As expected, the positive control dipyrone reduced the mechanical hyperalgesia induced by all of the stimuli [4, 46, 65]. These results suggest that AgD possess a mechanism of action different from dipyrone and has actions that occur between the activation of G-protein-coupled receptors and activation of the adenylyl-cyclase/cAMP pathway.
Some studies have shown that naphthoquinones might have antinociceptive/antiinflammatory activity through the inhibition of NF-κB activity. Ahn et al. showed that a furonaphthoquinone compound suppressed cyclooxygenase-2 expression in RAW 264.7 macrophages which may confer potential antiinflammatory activity to this compound [66]. Similarly, Song et al. showed that isoeleutherin suppressed the expression of inducible NO synthase and various cytokines by inhibiting NF-κB activity [67]. The effectiveness of AgD on PGE2-induced hyperalgesia suggests that it does not act by inhibiting NF-κB activity. To further support this hypothesis, we found that treating macrophages with AgD did not change the LPS-induced NO production at any of the concentrations tested. Therefore, AgD did not appear to exert a similar effect as the one observed for isoeleutherin [67].
Dipyrone has a specific antinociceptive effect on PGE2-induced hyperalgesia, which is not shared by most of the cyclooxygenase inhibitors, such as indomethacin. Lorenzetti & Ferreira and Duarte et al. showed that the peripheral effect of dipyrone was mediated by the activation of the L-arginine/NO/cGMP pathway [46, 65]. Subsequently, Alves & Duarte (2002) demonstrated that the antinociceptive effect of dipyrone in PGE2 induced mechanical hyperalgesia could be completely reversed by the local application of glibenclamide, an adenosine triphosphate-sensitive potassium channel blocker. In sharp contrast to dipyrone, the antinociceptive action of AgD was not reversed by glibenclamide, confirming that AgD does not act through this pathway.
The reduction of the paw withdrawal threshold induced by BK in mice may occur independently of cytokine release [2]. This peptide may cause nociception through its ability to directly activate nociceptors [68] by inducing the release of prostanoids and sympathetic amines [54, 61, 69] or binding to its G protein-coupled receptor B2 which is constitutively expressed in nociceptive fibers and promotes the activation of protein Gq/11, followed by the release of inositol triphosphate and diacylglycerol [68, 70]. The latter is responsible for nociceptive behavior by promoting the activation of protein kinase C, which can phosphorylate various ion channels [68, 71, 72]. Because AgD effectively reduced nociception induced by BK, we decided to test wether this compound acts on ion channels.
Transient receptor potential channels, one of the largest families of ion channels, are considered signal transducers that may participate in nociception [73]. We observed that AgD was ineffective in inhibiting nociceptive behavior induced by the TRPV1 channel activator capsaicin, TRPA1 channel activator cinnamaldehyde, ASIC3 channel activator acidic saline, and TRPM8 channel activator menthol [73]. Conversely, the nociceptive response produced by capsaicin was significantly reduced by ruthenium red, an inorganic polycationic dye that nonselectively blocks the response to several TRP channels. Additionally, the nociceptive responses induced by cinnamaldehyde and acidic saline were blocked by camphor and amiloride that are TRPA1 and ASIC antagonists, respectively.
In conclusion, the Esa had antinociceptive activity at doses that were not anti-pyretic or antiinflammatory. This antinociceptive activity was related, at least partially, to the presence of AgD, a new compound identified in this Sinningia species. AgD, at nanomolar doses signficantly reduced inflammatory pain by primarily preventing the sensitizing actions of the major mediators of inflammatory pain, such as cytokines, BK, PGE2, and dopamine. Its antinociceptive effect appeared to be peripheral and different from classic NSAIDs, glucocorticoids, opioids and dipyrone. AgD may interfere in some step between G-protein-coupled receptor activation and cAMP formation.
Supporting Information
Animals were treated with ethanolic extract form S. aggregata (ESa, 10, 30 and 100 mg/kg, as indicated), aggregatin D (AgD, 0.21 or 2.1 mg/kg) or the appropriate vehicles (Veh) by oral route or subcutaneously with diazepam (Dzp, 5 mg/kg) or fentanyl (Fent, 0.5 min). One hour after ESA or AgD or 15 min after Dzp or Fent animals were submitted to the rota-rod task (panel A) or hot-plate test (panel B). Data show the mean ± s.e.mean of the time spent in the rota-rod or the % of the maximal possible effect on the hot-plate test (n = 6–12). Symbols denote statistical difference in relation to Veh-treated group (***P<0.01).
(TIF)
Animals were treated with fractions petroleum ether (PE, 1.3 mg/kg), dichoromethane (DM, 1.5 mg/kg), ethyl acetate (EA, 7 mg/kg), indomethacin (Ind, 5 mg/kg) or vehicle (Veh), by oral route 1 h before the administration of formalin (2.5%, panel A) into the right paw Formalin-induced nociceptive behavior was evaluated in phase I (0–5 min) or in phase II (15 to 30 min). Bars represent the mean±s.e.mean of the nociceptive behavior (s) induced by formalin in each phase (n = 10–12). Symbols denote statistical difference in relation to veh-treated group (# P<0.05, ## P<0.01).
(TIF)
Acknowledgments
This study was supported by the Araucária Foundation of the State of Paraná and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Data Availability
All relevant data are within the paper.
Funding Statement
Araucária Foundation of the State of Paraná, Brazil # 413/09-15118 and Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil for the scholarships for GVS, ASS, ALBP, and JLCR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature. 413: 203–210. [DOI] [PubMed] [Google Scholar]
- 2. Cunha TM, Verri WA Jr, Fukada SY, Guerrero AT, Santodomingo-Garzon T, et al. (2007) TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor. Eur J Pharmacol. 573: 221–229. [DOI] [PubMed] [Google Scholar]
- 3. Denadai-Souza A, Camargo LL, Ribela MT, Keeble JE, Costa SK, et al. (2009) Participation of peripheral tachykinin NK1 receptors in the carrageenan-induced inflammation of the rat temporomandibular joint. Eur J Pain. 13: 812–819. 10.1016/j.ejpain.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 4. Cunha TM, Verri WA Jr, Silva JS, Poole S, Cunha FQ, et al. (2005) A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 102: 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decarie A, Adam A, Couture R (1996) Effects of captopril and Icatibant on bradykinin (BK) and des [Arg9] BK in carrageenan-induced edema. Peptides. 17: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 6. Gilligan JP, Lovato SJ, Erion MD, Jeng AY (1994) Modulation of carrageenan-induced hind paw edema by substance P. Inflammation. 18: 285–292. [DOI] [PubMed] [Google Scholar]
- 7. Mazzon E, Esposito E, Di Paola R, Muia C, Crisafulli C, et al. (2008) Effect of tumour necrosis factor-alpha receptor 1 genetic deletion on carrageenan-induced acute inflammation: a comparison with etanercept. Clin Exp Immunol. 153: 136–149. 10.1111/j.1365-2249.2008.03669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas G (1980) Characteristics of prostaglandin E1 potentiation of inflammatory activity of some agents. Prostaglandins. 19: 39–50. [DOI] [PubMed] [Google Scholar]
- 9.Werner U, Szelenyi I (1992) Measurement of MPO activity as model for detection of granulocyte infiltration in different tissues. Agents Actions Spec No: C101–103. [PubMed]
- 10. Roth J, De Souza GE (2001) Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 34: 301–314. [DOI] [PubMed] [Google Scholar]
- 11. Ferreira SH (1972) Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 240: 200–203. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, et al. (1997) Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 121: 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fine PG, Mahajan G, McPherson ML (2009) Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 10 Suppl 2: S79–88. 10.1111/j.1526-4637.2009.00666.x [DOI] [PubMed] [Google Scholar]
- 14. Zakrzewska JM (2010) Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother. 11: 1239–1254. 10.1517/14656561003767449 [DOI] [PubMed] [Google Scholar]
- 15. Vranken JH (2009) Mechanisms and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem. 9: 71–78. [DOI] [PubMed] [Google Scholar]
- 16. Chautems A, Lopes TCC, Peixoto M, Rossini J (2010) Taxonomic revision of Sinningia Nees (Gesneriaceae) IV: six new species from Brazil and a long overlooked taxon. Candollea 65: 241–266. [Google Scholar]
- 17. Swinny EE, Bloor SJ, Wong H (2000) H-1 and C-13 NMR assignments for the 3-deoxyanthocyanins, luteolinidin-5-glucoside and apigeninidin-5-glucoside. Magn Reson Chem 38: 1031–1033. [Google Scholar]
- 18. Verdan MH, Cervi AC, Campos FR, Barison A, Stefanello MEA (2009) Anthraquinones and ethylcyclohexane derivatives from Sinningia speciosa "Fyfiana". Biochem Syst Ecol. 37: 40–42. [Google Scholar]
- 19. Riva D, Barison A, Alves Stefanello ME, Poliquesi CB, Tasca Goes Ruiz AL, et al. (2012) Chemical study of Sinningia allagophylla guided by antiproliferative activity assays. Quim Nova. 35: 974–977. [Google Scholar]
- 20. Stefanello M, Cervi A, Wisniewsky A Jr (2005) Óleo essencial de Sinnigia aggregata Rev Bras Farmacogn. 15: 331–333. [Google Scholar]
- 21. Verdan MH, Barison A, de Sa EL, Salvador MJ, Poliquesi CB, et al. (2010) Lactones and Quinones from the Tubers of Sinningia aggregata. J Nat Prod 73: 1434–1437. 10.1021/np1002466 [DOI] [PubMed] [Google Scholar]
- 22.Barbosa FL, Mori LS, Riva D, Stefanello ME, Zampronio AR (2013) Antinociceptive and Anti-Inflammatory Activities of the Ethanolic Extract, Fractions and 8-Methoxylapachenol from Sinningia allagophylla Tubers. Basic Clin Pharmacol Toxicol. [DOI] [PubMed]
- 23. Luo P, Wong YF, Ge L, Zhang ZF, Liu Y, et al. (2010) Anti-inflammatory and Analgesic Effect of Plumbagin through Inhibition of Nuclear Factor-kappa B Activation. J Pharmacol Exp Ther. 335: 735–742. 10.1124/jpet.110.170852 [DOI] [PubMed] [Google Scholar]
- 24. Aoganghua A, Nishiumi S, Kobayashi K, Nishida M, Kuramochi K, et al. (2011) Inhibitory effects of vitamin K-3 derivatives on DNA polymerase and inflammatory activity. Int J Mol Med. 28: 937–945. 10.3892/ijmm.2011.773 [DOI] [PubMed] [Google Scholar]
- 25. Lu Y, Yang JH, Li X, Hwangbo K, Hwang S-L, et al. (2011) Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem Pharmacol. 82: 1700–1708. 10.1016/j.bcp.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 26. Kassuya CA, Cremoneze A, Barros LF, Simas AS, Lapa F da R, et al. (2009) Antipyretic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovata (Magnoliaceae). J Ethnopharmacol. 124: 369–376. 10.1016/j.jep.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 27. Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA (2005) Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol. 289: R1244–1252. [DOI] [PubMed] [Google Scholar]
- 28. De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26: 335–341. [DOI] [PubMed] [Google Scholar]
- 29. Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 30: 103–114. [DOI] [PubMed] [Google Scholar]
- 30. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 31. Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 32. Alves D, Duarte I (2002) Involvement of ATP-sensitive K(+) channels in the peripheral antinociceptive effect induced by dipyrone. Eur J Pharmacol, 444: 47–52. [DOI] [PubMed] [Google Scholar]
- 33. Otuki MF, Ferreira J, Lima FV, Meyre-Silva C, Malheiros A, et al. (2005) Antinociceptive properties of mixture of alpha-amyrin and beta-amyrin triterpenes: evidence for participation of protein kinase C and protein kinase A pathways. J Pharmacol Exp Ther. 313: 310–318. [DOI] [PubMed] [Google Scholar]
- 34. Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, et al. (2005) Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 115: 191–203. [DOI] [PubMed] [Google Scholar]
- 35. Cunha TM, Verri WA Jr, Vivancos GG, Moreira IF, Reis S, et al. (2004) An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37: 401–407. [DOI] [PubMed] [Google Scholar]
- 36. Moretao MP, Zampronio AR, Gorin PA, Iacomini M, Oliveira MB (2004) Induction of secretory and tumoricidal activities in peritoneal macrophages activated by an acidic heteropolysaccharide (ARAGAL) from the gum of Anadenanthera colubrina (Angico branco). Immunol Lett. 93: 189–197. [DOI] [PubMed] [Google Scholar]
- 37. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 126: 131–138. [DOI] [PubMed] [Google Scholar]
- 38. Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 39. Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K (1986) An improved colorimetric assay for interleukin 2. J Immunol Methods. 93: 157–165. [DOI] [PubMed] [Google Scholar]
- 40. Gasic GP, Hollmann M (1992) Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 54: 507–536. [DOI] [PubMed] [Google Scholar]
- 41. Baggio CH, Freitas CS, Marcon R, Werner MFD, Rae GA, et al. (2012) Antinociception of beta-D-glucan from Pleurotus pulmonarius is possibly related to protein kinase C inhibition. Int J Biol Macromol. 50: 872–877. 10.1016/j.ijbiomac.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 42. Andrade EL, Luiz AP, Ferreira J, Calixto JB (2008) Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience 152: 511–520. 10.1016/j.neuroscience.2007.12.039 [DOI] [PubMed] [Google Scholar]
- 43. Amir M, Kumar S (2007) Synthesis and evaluation of anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation properties of ibuprofen derivatives. Acta Pharm. 57: 31–45. [DOI] [PubMed] [Google Scholar]
- 44. Perretti M, Flower RJ (1994) Cytokines, glucocorticoids and lipocortins in the control of neutrophil migration. Pharmacol Res. 30: 53–59. [DOI] [PubMed] [Google Scholar]
- 45. Coelho MM, Souza GE, Pela IR (1992) Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol. 263: R423–427. [DOI] [PubMed] [Google Scholar]
- 46. Lorenzetti BB, Ferreira SH (1985) Mode of analgesic action of dipyrone: direct antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 114: 375–381. [DOI] [PubMed] [Google Scholar]
- 47. al-Swayeh OA, Futter LE, Clifford RH, Moore PK (2000) Nitroparacetamol exhibits anti-inflammatory and anti-nociceptive activity. Br J Pharmacol. 130: 1453–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanashiro A, Pessini AC, Machado RR, Malvar D do C, Aguiar FA, et al. (2009) Characterization and pharmacological evaluation of febrile response on zymosan-induced arthritis in rats. Am J Physiol. 296: R1631–1640. 10.1152/ajpregu.90527.2008 [DOI] [PubMed] [Google Scholar]
- 49. De Souza GE, Cardoso RA, Melo MC, Fabricio AS, Silva VM, et al. (2002) A comparative study of the antipyretic effects of indomethacin and dipyrone in rats. Inflamm Res. 51: 24–32. [DOI] [PubMed] [Google Scholar]
- 50. Quintao NL, Medeiros R, Santos AR, Campos MM, Calixto JB (2005) The effects of diacerhein on mechanical allodynia in inflammatory and neuropathic models of nociception in mice. Anesth Analg. 101: 1763–1769. [DOI] [PubMed] [Google Scholar]
- 51. Gao Y, Liu H, Deng L, Zhu G, Xu C, et al. (2011) Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull. 84: 406–413. 10.1016/j.brainresbull.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 52. Villasenor IM, Angelada J, Canlas AP, Echegoyen D (2002) Bioactivity studies on beta-sitosterol and its glucoside. Phytother Res. 16: 417–421. [DOI] [PubMed] [Google Scholar]
- 53. Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, et al. (2006) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther.112: 116–138. [DOI] [PubMed] [Google Scholar]
- 54. Ferreira SH (1993) The role of interleukins and nitric oxide in the mediation of inflammatory pain and its control by peripheral analgesics. Drugs 46 Suppl 1: 1–9. [DOI] [PubMed] [Google Scholar]
- 55. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH (1992) The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 107: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Oliveira Fusaro MC, Pelegrini-da-Silva A, Araldi D, Parada CA, Tambeli CH (2010) P2X3 and P2X2/3 receptors mediate mechanical hyperalgesia induced by bradykinin, but not by pro-inflammatory cytokines, PGE(2) or dopamine. Eur J Pharmacol. 649: 177–182. 10.1016/j.ejphar.2010.09.037 [DOI] [PubMed] [Google Scholar]
- 57. Southall MD, Vasko MR (2001) Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 276: 16083–16091. [DOI] [PubMed] [Google Scholar]
- 58. Narumiya S (2009) Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med. (Berl) 87: 1015–1022. 10.1007/s00109-009-0500-1 [DOI] [PubMed] [Google Scholar]
- 59. Coderre TJ, Abbott FV, Melzack R (1984) Effects of peripheral antisympathetic treatments in the tail-flick, formalin and autotomy tests. Pain. 18: 13–23. [DOI] [PubMed] [Google Scholar]
- 60. Duarte ID, Nakamura M, Ferreira SH (1988) Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res 21: 341–343. [PubMed] [Google Scholar]
- 61. Khasar SG, Miao JP, Janig W, Levine JD (1998) Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur J Neurosci. 10: 435–444. [DOI] [PubMed] [Google Scholar]
- 62. Villarreal CF, Funez MI, Cunha FQ, Parada CA, Ferreira SH (2013) The long-lasting sensitization of primary afferent nociceptors induced by inflammation involves prostanoid and dopaminergic systems in mice. Pharmacol Biochem Behav. 103: 678–683. 10.1016/j.pbb.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 63. Memo M, Missale C, Carruba MO, Spano PF (1986) Pharmacology and biochemistry of dopamine receptors in the central nervous system and peripheral tissue. J Neural Transm. Suppl 22: 19–32. [PubMed] [Google Scholar]
- 64. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev. 78: 189–225. [DOI] [PubMed] [Google Scholar]
- 65. Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH (1992) Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 217: 225–227. [DOI] [PubMed] [Google Scholar]
- 66. Ahn KY, Kim BH, Lee YR, Hwang DH, Chung EY, et al. (2005) Dual inhibitory effects of furonaphthoquinone compound on enzyme activity and lipopolysaccharide-induced expression of cyclooxygenase-2 in macrophages. Biochem Biophys Res Commun. 336: 93–99. [DOI] [PubMed] [Google Scholar]
- 67. Song SH, Min HY, Han AR, Nam JW, Seo EK, et al. (2009) Suppression of inducible nitric oxide synthase by (-)-isoeleutherin from the bulbs of Eleutherine americana through the regulation of NF-kappaB activity. Int Immunopharmacol. 9: 298–302. 10.1016/j.intimp.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 68. Petho G, Reeh PW (2012) Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 92: 1699–1775. 10.1152/physrev.00048.2010 [DOI] [PubMed] [Google Scholar]
- 69. Taiwo YO, Levine JD (1988) Characterization of the arachidonic acid metabolites mediating bradykinin and noradrenaline hyperalgesia. Brain Res. 458: 402–406. [DOI] [PubMed] [Google Scholar]
- 70. Dray A, Bettaney J, Forster P, Perkins MN (1988) Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neurosci Lett. 91: 301–307. [DOI] [PubMed] [Google Scholar]
- 71. Barber LA, Vasko MR (1996) Activation of protein kinase C augments peptide release from rat sensory neurons. J Neurochem. 67: 72–80. [DOI] [PubMed] [Google Scholar]
- 72. Gutowski S, Smrcka A, Nowak L, Wu DG, Simon M, et al. (1991) Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 266: 20519–20524. [PubMed] [Google Scholar]
- 73. Levine JD, Alessandri-Haber N (2007) TRP channels: targets for the relief of pain. Biochim Biophys Acta. 1772: 989–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animals were treated with ethanolic extract form S. aggregata (ESa, 10, 30 and 100 mg/kg, as indicated), aggregatin D (AgD, 0.21 or 2.1 mg/kg) or the appropriate vehicles (Veh) by oral route or subcutaneously with diazepam (Dzp, 5 mg/kg) or fentanyl (Fent, 0.5 min). One hour after ESA or AgD or 15 min after Dzp or Fent animals were submitted to the rota-rod task (panel A) or hot-plate test (panel B). Data show the mean ± s.e.mean of the time spent in the rota-rod or the % of the maximal possible effect on the hot-plate test (n = 6–12). Symbols denote statistical difference in relation to Veh-treated group (***P<0.01).
(TIF)
Animals were treated with fractions petroleum ether (PE, 1.3 mg/kg), dichoromethane (DM, 1.5 mg/kg), ethyl acetate (EA, 7 mg/kg), indomethacin (Ind, 5 mg/kg) or vehicle (Veh), by oral route 1 h before the administration of formalin (2.5%, panel A) into the right paw Formalin-induced nociceptive behavior was evaluated in phase I (0–5 min) or in phase II (15 to 30 min). Bars represent the mean±s.e.mean of the nociceptive behavior (s) induced by formalin in each phase (n = 10–12). Symbols denote statistical difference in relation to veh-treated group (# P<0.05, ## P<0.01).
(TIF)
Data Availability Statement
All relevant data are within the paper.