Abstract
Objective
To determine risk factors for cesarean delivery in nulliparas at labor admission.
Study Design
Nulliparas with liveborn, singleton gestations ≥37 weeks in spontaneous or induced labor were analyzed from the Consortium on Safe Labor database in a retrospective observational study. Classification and regression tree (CART) and multivariate logistic regression analysis determined risk factors for cesarean delivery.
Result
Of the 66,539 nulliparas, 22% had a cesarean delivery. In the CART analysis, the first cervical dilation exam was the first branch followed by body mass index (BMI). Cesarean deliveries occurred in 45%, 25%, 14%, and 10% of deliveries at <1cm, 1-3cm, 4cm, and ≥5cm dilated respectively. The BMI influence was most evident in the <1cm dilation category with 26% of BMI<25 and 66% of BMI≥40 having a cesarean delivery. The fewest cesarean deliveries (5%) occurred in those ≥5cm and BMI<25. In the multivariate regression analysis, first cervical dilation exam <1cm (OR 5.1, 95%CI 4.5-5.7; reference ≥5cm) and BMI≥40 (OR 5.1, 95%CI 4.6-5.7; reference BMI<25.0) had the highest odds for cesarean delivery.
Conclusion
Cervical dilation on admission followed by BMI were the two most important risk factors for cesarean delivery identified in both CART and multivariate regression analysis.
Keywords: Classification and regression tree analysis, Cesarean delivery, Nulliparas
Introduction
A critical issue in obstetrics is the rising cesarean delivery rate, which reached a high at 32.9% in 2009 in the United States.1 Nulliparas comprise approximately 22-26% of all cesarean deliveries.1, 2 In addition to the known short-term complications such as infectious morbidity and thromboembolic events, cesarean deliveries are associated with long-term complications such as abnormal placentation and hysterectomy.3, 4 The cesarean performed in a nullipara is a seminal event as it impacts future delivery route decisions and potentially contributes to maternal morbidity.
The ability to determine which women are at higher risk for cesarean delivery is important in obstetrical care as this may modify management protocols and lead to improved perinatal outcomes. This is especially true for nulliparas as labor course and outcome is more difficult to determine compared to multiparas. Logistic regression analyses are amongst the most common methods used to describe associations between risk factors and outcomes (e.g., trial of labor after cesarean delivery and delivery route), but they have limited ability to predict outcomes.5, 6, 7, 8, 9 Classification and regression tree (CART) analysis not only accounts for complex relationships between variables, but also presents the results in a clinically useful form. In this approach, progressive splitting of the study population into subgroups occurs according to the independent variables. The underlying mathematical algorithm chooses the variables to split by, the discriminatory values of the variables, and the order in which splitting occurs, with the objective of maximizing outcome discrimination at each step. The process develops a hierarchical tree structure that allows for the visualization of complex interactions when multiple variables are analyzed simultaneously. CART analysis is a different approach to studying risk factors and outcomes and a few investigations in obstetrics (e.g., outcomes after emergent cerclage, perineal lacerations, postpartum hemorrhage, and primary cesarean delivery in a non-laboring population) have employed this methodology.8, 10, 11, 12 The objective of this study was to identify factors present upon labor admission that are associated with cesarean delivery in nulliparas from the Consortium on Safe Labor database using CART analysis and then compare these findings to multivariate logistic regression analysis.
Materials and Methods
This is a secondary analysis of data from the Consortium on Safe Labor, which sought to establish a comprehensive database from multiple sites and characterize labor and delivery in a contemporary group of women experiencing current obstetrical clinical practices. The complete database contained 228,438 deliveries between 2002 and 2008 acquired from electronic obstetrical databases. Twelve clinical centers with 19 distinct hospitals across 9 American College of Obstetricians and Gynecologists (ACOG) districts participated in the Consortium on Safe Labor. The majority (87%) of births occurred between 2005 and 2007. All live births at 23 weeks or later were included in the database. Participating institutions extracted detailed information from their electronic medical records on maternal demographics, medical history, reproductive and prenatal history, labor and delivery, postpartum, and newborn information. The Institutional Review Boards of all 12 participating institutions approved the initial project. More detailed information regarding the formation of the database is provided elsewhere.13, 14
For the current retrospective observational study, the inclusion criteria were nulliparas with live-born cephalic singletons at ≥ 37 0/7 weeks gestation. Excluded were women with a prelabor cesarean delivery, defined by an absent trial of labor and a first cervical dilation exam <6cm and none of the following criteria: induction of labor, augmentation of labor, intrapartum tocolytics, episiotomy, shoulder dystocia, vaginal lacerations, a cesarean performed for failure to progress or failed induction, or a date/time of full cervical dilation or onset of spontaneous labor in the database. All other cesarean deliveries not meeting the above criteria were classified as intrapartum and included in the study. A nullipara was a woman without a prior 20 week or longer gestational age delivery. The primary outcome was delivery route categorized as cesarean or vaginal delivery. Independent variables considered included maternal age, race, gestational age, short stature (<1.5 m), admission body mass index (BMI, kg/m2), pre-gestational or gestational diabetes, hypertension (all types combined), cervical dilation on admission or first cervical exam recorded (in centimeters), and induction of labor. The self-reported maternal height and weight most proximate to the time of labor admission (last documented weight at a prenatal visit or admission weight) were used to calculate BMI for each patient. These independent variables were selected not only because they were known at the time of admission to labor and delivery, but also because they have been shown to be associated with delivery route.15, 16, 17, 18, 19, 20 The data for maternal age (< 30 vs. ≥ 30 years), gestational age (37-39 vs. ≥ 40 weeks), height (<1.5 m), and admission or first cervical dilation (<1 cm, 1-3 cm, 4 cm, or ≥ 5 cm) were analyzed as categorical variables based on the distribution of the variables in the database and clinical relevance. Any further reference to cervical dilation reflects either the admission or first cervical exam in the database. The BMI data were grouped into categories by 5 unit increments, similar to WHO criteria (< 25.0 kg/m2, 25.0-29.9 kg/m2, 30.0-34.9 kg/m2, 35.0-39.9 kg/m2, and ≥ 40 kg/m2).21 These independent variables were compared between the cesarean and vaginal deliveries using two sided t-tests for continuous and chi-square tests for categorical variables. A P-value <0.05 was considered statistically significant.
CART analysis then studied the dependent variable of delivery route with the independent variables described above using IBM SPSS Decision Trees 22 (Chicago, IL), implementing the Exhaustive Chi-squared Automatic Interaction Detection methodology, with 10-fold cross-validation. This cross-validation procedure allows use of all the data, where the dataset is randomly divided into 10 separate sub-groups of participants. For each of the 10 sub-groups, a tree is built with the 90% of the participants, also known as the learning sample. The 10% sub-group is then treated as a test sample. Each of the 10% sub-groups serve once as a test sample and serve as part of the learning sample 9 times. A total of 10 trees are built with each one excluding one of the sub-groups, with the final classification tree based on a summary of the 10 separate classification trees. Further specification of the classification tree included an alpha of 0.05 for splitting after incorporating a Bonferonni adjustment, a minimum parent node size of 1000 cases, a minimum child node size of 500 cases, and a maximum tree depth of 3. Separate analysis which excluded labor inductions and missing data were also performed. Next, univariate analysis followed by multivariate logistic regression analysis was done using a backward elimination model with the same independent and dependent variables as the CART analysis. Odds ratios (OR) and 95% confidence intervals (CI) were reported.
Results
Of the 228, 438 deliveries in the entire Consortium on Safe Labor database, 91,424 (40%) were nulliparas. After exclusions, 66,539 met the eligibility criteria (Figure 1). Table 1 describes the demographic data of the eligible cohort grouped by delivery route where 22% overall had a cesarean delivery. Short stature, African American and Hispanic race/ethnicity, diabetes, hypertension, and induced labor were more common in cesarean compared to vaginal deliveries, P<0.001. Greater mean maternal age (26.6±6.4 vs. 24.5±5.7 years), and admission BMI (32.6±6.8 vs. 29.6±5.4 kg/m2) were also characteristic of the cesarean delivery group, P<0.001. In addition, those delivered by cesarean had a lower cervical dilation (2.2±1.8 cm) compared to vaginal deliveries (3.3±2.2 cm, P<0.001).
Figure 1. Participant selection.
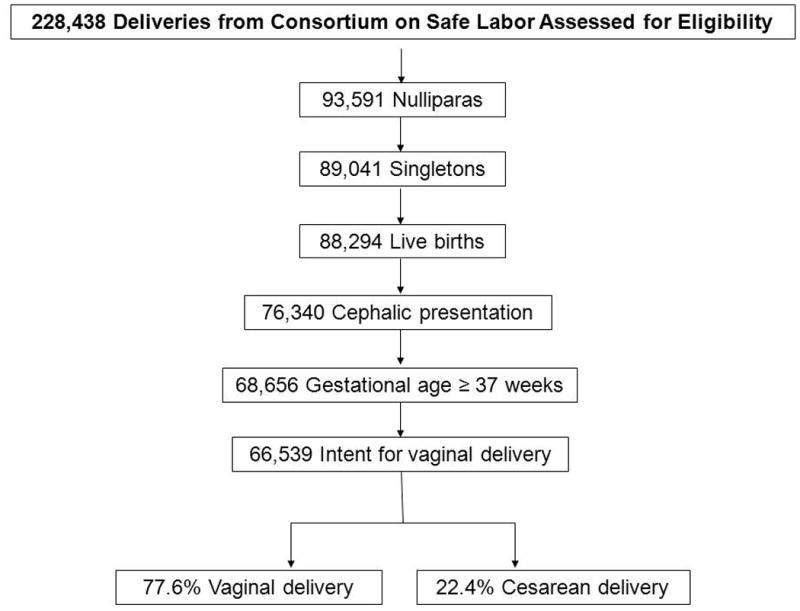
Table 1. Demographics, Medical History, and Labor Characteristics1.
| Total | Delivery Mode | ||||||
|---|---|---|---|---|---|---|---|
| Cesarean | Vaginal | ||||||
| N or mean | % or SD | N or mean | % or SD | N or mean | % or SD | ||
| Variable | 14883 | 22.4 | 51656 | 77.6 | |||
| Maternal Age (years) | <30 | 51202 | 77.0 | 9954 | 66.9 | 41248 | 79.9 |
| ≥30 | 15263 | 23.0 | 4913 | 33.1 | 10350 | 20.1 | |
| Mean ± SD | 25.0 | 6.0 | 26.6 | 6.5 | 24.5 | 5.7 | |
| Maternal Height (meters) | <1.5 | 1427 | 2.5 | 521 | 3.5 | 906 | 1.7 |
| Mean ± SD | 1.63 | 0.07 | 1.62 | 0.07 | 1.64 | 0.07 | |
| Missing | 9240 | 13.9 | 2085 | 14.0 | 7152 | 13.8 | |
| Maternal Race | White | 33229 | 52.6 | 6361 | 44.7 | 26868 | 54.9 |
| Black | 13847 | 21.9 | 3821 | 26.8 | 10026 | 20.5 | |
| Hispanic | 10858 | 17.2 | 2707 | 19.0 | 8151 | 16.7 | |
| Other | 5231 | 8.3 | 1347 | 9.5 | 3884 | 7.9 | |
| Missing | 3374 | 5.1 | 647 | 1.0 | 2727 | 4.1 | |
| Body Mass Index Category | <25.0 | 8681 | 13.1 | 984 | 6.6 | 7697 | 14.9 |
| 25.0-29.9 | 22470 | 33.8 | 4002 | 26.9 | 18468 | 35.7 | |
| 30.0-34.9 | 14019 | 21.1 | 1940 | 23.9 | 3864 | 20.2 | |
| 35.0-39.9 | 5804 | 8.7 | 1658 | 13.0 | 2096 | 7.5 | |
| ≥40.0 | 3754 | 5.6 | 1658 | 11.1 | 2096 | 4.1 | |
| Missing | 11811 | 17.7 | 2735 | 18.4 | 9076 | 17.6 | |
| Mean ± SD | 30.3 | 5.9 | 32.7 | 6.9 | 29.6 | 5.4 | |
| Any diabetes | 2377 | 3.6 | 908 | 6.1 | 1469 | 2.8 | |
| Any hypertension | 3444 | 5.2 | 1162 | 7.8 | 2282 | 4.4 | |
| Gestational Age (weeks) | 37-39 weeks | 39152 | 58.8 | 7492 | 50.3 | 31660 | 61.3 |
| ≥40 weeks | 27387 | 41.2 | 7391 | 49.7 | 19996 | 38.7 | |
| Mean ± SD | 39.4 | 1.1 | 39.6 | 1.2 | 39.4 | 1.1 | |
| Admission or first cervical dilation (cm) | <1 cm | 6359 | 10.9 | 2863 | 19.2 | 3496 | 6.8 |
| 1-3 cm | 25777 | 44.2 | 6576 | 44.1 | 19207 | 37.2 | |
| 4 cm | 21869 | 32.9 | 3164 | 21.3 | 18705 | 36.2 | |
| ≥5 cm | 8277 | 12.4 | 778 | 5.2 | 7499 | 14.5 | |
| Missing | 4257 | 7.3 | 1508 | 10.1 | 2749 | 5.3 | |
| Mean ± SD | 2.7 | 1.9 | 1.9 | 1.6 | 3.0 | 2.0 | |
| Induction of labor | 29492 | 44.3 | 9156 | 61.5 | 20336 | 39.4 | |
SD standard deviation
The P-value was <0.001 for all comparisons between cesarean and vaginal deliveries.
Missing data accounted for 74 (0.1%) maternal age, 3,374 (5%) maternal race, 11,811 (17%) admission BMI's, and 4,257 (6.4%) cervical dilations, with some women having more than one of the variables missing. For the CART analysis, a separate “missing” branch was created for the age and race variables, leaving 51,104 participants for this analysis. Cervical dilation was the first branch of the CART. Cesarean deliveries occurred in 45%, 25%, 14%, and 10% at <1 cm, 1-3 cm, 4 cm, and ≥5 cm respectively (Figure 2). BMI was the second branch for all dilation categories. A BMI≥30 was associated with the most cesarean deliveries across all dilation levels. The BMI influence was most evident in the <1cm dilation category with 26% of BMI < 25 and 66% of BMI ≥ 40 having a cesarean delivery. The terminal nodes were varying occurrences of maternal age, gestational age, and race but none of them represented extreme occurrences of cesarean delivery (data not shown). The fewest cesareans (5%) occurred in those ≥5 cm and BMI < 25. Height, diabetes, hypertension, and induction did not present as significant contributors over and above the other independent variables included in this model.
Figure 2. Classification and Regression Tree Analysis for Delivery Route in Nulliparas.
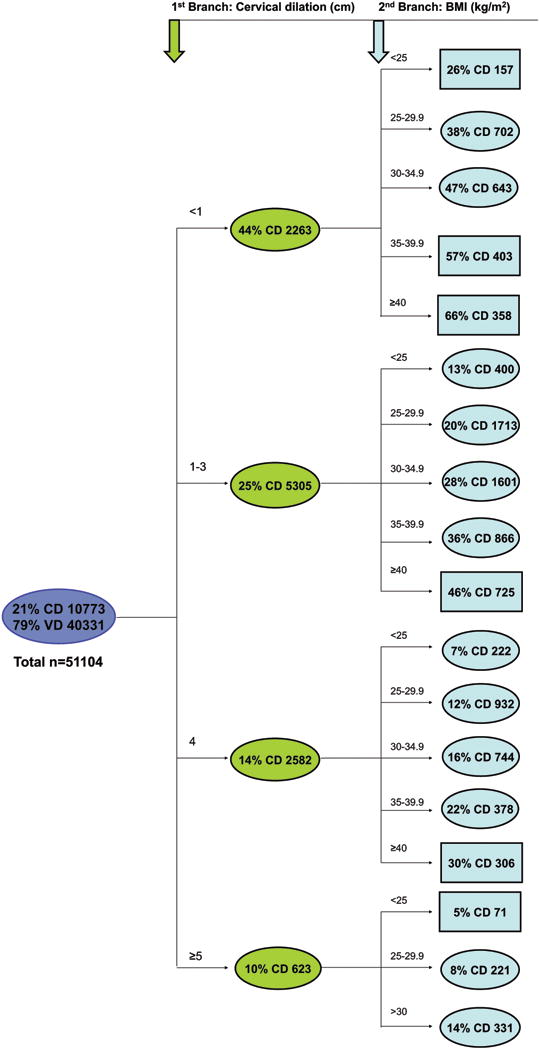
CD cesarean delivery, VD vaginal delivery, GA gestational age
Data presented as CD% and number in each shape (ovals and rectangles)
Rectangles represent terminal nodes (no other variables had a statistically significant impact on delivery route).
3rd branches were variations of maternal age, gestational age, or race, but none represented extreme occurrences of cesarean delivery (data not shown).
Because of the potential correlation between cervical dilation and induction (i.e., more inductions at lower cervical dilations), the induction variable was removed and the analysis was repeated. All branch points and proportions of cesarean deliveries were exactly the same as the analysis with induction (data not shown) which again confirmed that induction was not one of the top hierarchical variables. Maternal age, height, and gestational age were also entered into the analysis as continuous variables. The first two branch points (cervical dilation and BMI) and cesarean delivery proportions were exactly the same with varying occurrences of maternal age, height, and gestational age in the third branches (data not shown). To assess the impact of missing data, another analysis was performed whereby a “missing” value was entered as a separate category for cervical dilation and BMI. The results were similar with a first branch point of cervical dilation followed by second branch points of either BMI or induction and then varying occurrences of maternal age, gestational age, and BMI as the third branch points (results not shown). The highest proportion of cesarean deliveries still occurred in women with a cervical dilation <1 cm and BMI ≥ 40 (66%) whereas the fewest occurred in cervical dilation ≥5 cm, <30 years old and not induced (7%). The “missing” cervical dilation (35% cesarean delivery) branch was further split by induction whereby 47% of induced labors resulted in a cesarean delivery.
In the univariate and multivariate logistic regression analysis, all variables were significantly associated with delivery route except for hypertension in the multivariate analysis (Table 2). Cervical dilation <1cm (OR 5.1 95% CI 4.5-5.7; reference ≥5cm) and BMI≥ 40 (OR 5.1, 95% CI 4.6-5.7; reference <25.0) had the highest odds for cesarean delivery in the multivariate analysis.
Table 2. Univariate and Multivariate logistic regression analysis for cesarean delivery.
| Variable | Unadjusted OR | 95%CI | Adjusted OR 1 | 95%CI |
|---|---|---|---|---|
| Maternal age ≥30 years | 2.0 | 1.9-2.1 | 1.9 | 1.8-2.0 |
| Maternal height <1.5m | 2.0 | 1.8-2.3 | 1.9 | 1.7-2.2 |
| Race | ||||
| White | ref | ref | ||
| Black | 1.6 | 1.5-1.7 | 1.7 | 1.6-1.8 |
| Hispanic | 1.4 | 1.3-1.5 | 1.5 | 1.4-1.6 |
| Other | 1.5 | 1.4-1.6 | 1.6 | 1.5-1.8 |
| Admission BMI (kg/m2) | ||||
| <25.0 | ref | ref | ||
| 25.0-29.9 | 1.7 | 1.6-1.8 | 1.6 | 1.5-1.7 |
| 30.0-34.9 | 2.7 | 2.5-2.9 | 2.5 | 2.3-2.7 |
| 35.0-39.9 | 3.9 | 3.6-4.3 | 3.5 | 3.2-3.9 |
| ≥40.0 | 6.2 | 5.6-6.8 | 5.1 | 4.6-5.7 |
| Diabetes | 2.2 | 2.0-2.4 | 1.4 | 1.3-1.6 |
| Hypertension | 1.8 | 1.7-2.0 | --2 | -- |
| Gestational age >40 weeks | 1.6 | 1.5-1.6 | 1.5 | 1.4-1.6 |
| Admission cervical dilation | ||||
| <1 cm | 7.9 | 7.2-8.6 | 5.1 | 4.5-5.7 |
| 1-3 cm | 3.3 | 3.1-3.6 | 2.6 | 2.4-2.9 |
| 4 cm | 1.6 | 1.5-1.8 | 1.5 | 1.4-1.6 |
| ≥5 cm | ref | ref | ||
| Induction | 2.5 | 2.4-2.6 | 1.4 | 1.3-1.4 |
The multivariate model included all the covariates specified in the ‘Adjusted OR’ column of this table.
Hypertension was eliminated from the final model in the backward elimination regression analysis.
Discussion
In this large multicenter retrospective investigation using an electronic obstetrical database, we determined a hierarchy of risk factors for cesarean delivery from an analysis of maternal demographics, medical history, and labor characteristics in nulliparas based on information available when the woman was admitted to labor and delivery. The CART analysis indicated that the most discriminatory risk factor for cesarean delivery was cervical dilation, followed by admission BMI. Similar to prior studies, other demographic and clinical factors also influenced cesarean deliveries whereby maternal age, race, and gestational age were the most important ones in the current study.15, 16, 17, 19 This information is timely as cesarean deliveries continue to climb, maternal weight is increasing in parallel with the obesity epidemic in the United States, and other collaborations have begun to implement protocols and initiatives to reduce elective inductions and unplanned cesarean deliveries along with improving the overall quality of care.22, 23, 24 Our number of intrapartum cesarean deliveries (22%) was higher than reports from Ireland for nulliparous women with a singleton cephalic fetus at term (14%), but similar to overall cesarean deliveries for nulliparous women in the United States (22-26%).2, 15, 25 Other studies also support that expectant management of nulliparas could lead to lower cesarean deliveries, yet there is also evidence to support that elective inductions decrease cesarean deliveries and other perinatal morbidity.15, 26, 27, 28, 29 This will likely be an ongoing area of population-based research. In the interim, according to the summary from the “Preventing the First Cesarean Delivery” workshop in 2012, labor induction with an unfavorable cervix should not be undertaken unless delivery is indicated for clear maternal or fetal benefit.30
The advantage of using CART methodology as compared to multivariate logistic regression was the clearer delineation of the most important risk factors for cesarean delivery among several variables that have known associations with delivery route. The CART analysis also allows for a more visual presentation of the data compared to interpreting coefficients from logistic regression models, and so clinicians and patients may have a better understanding of their labor status and outcomes if they are explained with the CART analysis method as compared to odds ratios or relative risks. For example, based on the CART analysis in this study, 26-66% of nulliparas with a cervical exam of < 1cm had a cesarean delivery, as compared to lower cesarean delivery percentages (7-18%) if the admission occurred when the cervix was at least 4-5 cm dilated, depending on values of other important factors such as BMI and maternal age. From the logistic regression analysis, there is a 5.1-fold increased odds for cesarean delivery if admitted at 1 cm compared with a dilation of 5cm or more (Table 2), assuming all other factors in the model are held constant. This finding may be less interpretable for a layperson.
Although not all deliveries from the entire Consortium of Safe Labor cohort were included in this study, this secondary analysis was similar with respect to demographics, BMI, and inductions to the overall cohort.13, 14 The multicenter approach allows for the information to be generalized to practices across the United States, but especially so for those who manage labor of obese women. As a retrospective study, individual cervical exams, labor practices, and indications for cesarean delivery could not be assessed, but the outcomes in this database likely reflect actual decision-making and clinical practice. Approaches to cervical ripening included a full-spectrum of agents including prostaglandins and foley bulbs, etc. We opted to use admission BMI rather than a prepregnancy BMI because weight most proximate to delivery would have a greater impact on actual delivery route. The cervical dilation variable was a combination of the cervical exam on admission (mean 2.98 ± 2.16 cm) and the first cervical exam (mean 2.74 ± 1.95 cm) in the database which minimized the missing data for this variable but may have introduced some bias with higher cervical dilations being analyzed. As the intent was to study factors present at admission, intrapartum variables such as oxytocin, chorioamnionitis, epidurals, labor length, or birth weight that may have also influenced the delivery route were not included in the analysis.
In large databases such as this one, it is expected to have missing data. This was minimal in most analyzed variables except for BMI. A unique feature of CART is that it allows for analysis of missing data as a discrete category. We can explain the differences in delivery route in the “missing” cervical dilation category by assuming that those who were induced had a low cervical dilation (i.e. <1 cm) and therefore higher cesareans (47%). Likewise, those who presented with advanced dilations and delivered vaginally (80% of non-induced missing cervical dilations) and quickly after admission did not have a documented cervical exam. With respect to missing BMI, there did not appear to be a trend to this missing data as cesarean delivery for missing BMI fell in between the other BMI categories in each cervical dilation branch suggesting that the missing BMI data represented the full range of BMI. Given the otherwise similar findings of the two CART analysis (with and without missing data categories), we do not suspect that missing data entered appreciable bias in the study results.
Although most nulliparas will have a vaginal delivery, this study provides clinically useful information that may not only help determine nulliparas who will require a cesarean delivery by using easily ascertainable clinical characteristics available at the time of admission but also assist clinicians in counseling patients about the risk for cesarean and provide further data to encourage nulliparas to await spontaneous labor if there are no contraindications, or to delay admission until the cervix is dilated to at least 4 cm, as deterrents to cesarean delivery. Future studies are needed to investigate more accurate analytical approaches to identify risk factors for the first cesarean delivery, yet our CART analysis findings were similar to those of logistic regression analysis. Further studies should also consider using CART analysis as an alternative to more traditional regression analysis to determine the most important risk factors for other perinatal outcomes.
Acknowledgments
The data included in this paper were obtained from the Consortium on Safe Labor, which was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C. Institutions involved in the Consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. The named authors alone are responsible for the views expressed in this manuscript, which does not necessarily represent the decisions or the stated policy of the NICHD.
This study was supported by (1) the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through a contract (Contract No. HHSN267200603425C) for the original Consortium on Safe Labor study, (2) Grant Number K23 HD076010 from the NICHD and NIH (author MK), and (3) University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources.
Footnotes
This study was presented in part as a poster at the Annual Meeting for the Society of Maternal Fetal Medicine in San Francisco, California on February 11, 2011.
Conflict of interest: Drs. Kominiarek, VanVeldhuisen, Gregory, and Hibbard all received funding from the NIH/NICHD for the original Consortium on Safe Labor project. Ms. Kim and Dr. Fridman declare no potential conflict of interest. Dr. Kominiarek currently has a K23 Mentored Career Development Award from the NIH/NICHD.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 2.Coonrod DV, Drachman D, Hobson P, Manriquez M. Nulliparous term singleton vertex cesarean delivery rates: institutional and individual level predictors. Am J Obstet Gynecol. 2008;198(6):694 e691–611. doi: 10.1016/j.ajog.2008.03.026. discussion 694 e611. [DOI] [PubMed] [Google Scholar]
- 3.Makoha FW, Felimban HM, Fathuddien MA, Roomi F, Ghabra T. Multiple cesarean section morbidity. Internat J Gynaecol Obstet. 2004;87(3):227–232. doi: 10.1016/j.ijgo.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecology. 2006;107(6):1226–1232. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 5.Eden KB, McDonagh M, Denman MA, Marshall N, Emeis C, Fu R, et al. New insights on vaginal birth after cesarean: can it be predicted? Obstet Gynecol. 2010;116(4):967–981. doi: 10.1097/AOG.0b013e3181f2de49. [DOI] [PubMed] [Google Scholar]
- 6.Flamm BL, Geiger AM. Vaginal birth after cesarean delivery: an admission scoring system. Obstet Gynecol. 1997;90(6):907–910. doi: 10.1016/s0029-7844(97)00531-0. [DOI] [PubMed] [Google Scholar]
- 7.Grobman WA, Lai Y, Landon MB, Spong CY, Leveno KJ, Rouse DJ, et al. Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;109(4):806–812. doi: 10.1097/01.AOG.0000259312.36053.02. [DOI] [PubMed] [Google Scholar]
- 8.Grobman WA, Terkildsen MF, Soltysik RC, Yarnold PR. Predicting outcome after emergent cerclage using classification tree analysis. Am J Perinatol. 2008;25(7):443–448. doi: 10.1055/s-0028-1083843. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt MG, Martin JN, Jr, Meydrech EF, Blake PG, Martin RW, Perry KG, Jr et al. Vaginal birth after cesarean delivery: are there useful and valid predictors of success or failure? Am J Obstet Gynecol. 1992;166(6 Pt 1):1811–1815. doi: 10.1016/0002-9378(92)91572-r. discussion 1815-1819. [DOI] [PubMed] [Google Scholar]
- 10.Gregory KD, Korst LM, Platt LD. Variation in elective primary cesarean delivery by patient and hospital factors. Am J Obstet Gynecol. 2001;184(7):1521–1532. doi: 10.1067/mob.2001.115496. discussion 1532-1524. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton EF, Smith S, Yang L, Warrick P, Ciampi A. Third- and fourth-degree perineal lacerations: defining high-risk clinical clusters. Am J Obstet Gynecol. 2011;204(4):309 e301–306. doi: 10.1016/j.ajog.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Lu MC, Korst LM, Fridman M, Muthengi E, Gregory KD. Identifying women most likely to benefit from prevention strategies for postpartum hemorrhage. J Perinatol. 2009;29(6):422–427. doi: 10.1038/jp.2009.2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–1287. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326 e321–326 e310. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan DJ, Murphy M, Robson MS, O'Herlihy C. The singleton, cephalic, nulliparous woman after 36 weeks of gestation: contribution to overall cesarean delivery rates. Obstet Gynecol. 2011;117(2 Pt 1):273–279. doi: 10.1097/AOG.0b013e318204521a. [DOI] [PubMed] [Google Scholar]
- 16.Heffner LJ, Elkin E, Fretts RC. Impact of labor induction, gestational age, and maternal age on cesarean delivery rates. Obstet Gynecol. 2003;102(2):287–293. doi: 10.1016/s0029-7844(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 17.Kominiarek MA, Vanveldhuisen P, Hibbard J, Landy H, Haberman S, Learman L, et al. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203(3):264 e261–267. doi: 10.1016/j.ajog.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch CM, Sexton DJ, Hession M, Morrison JJ. Obesity and mode of delivery in primigravid and multigravid women. Am J Perinatol. 2008;25(3):163–167. doi: 10.1055/s-2008-1061496. [DOI] [PubMed] [Google Scholar]
- 19.Treacy A, Robson M, O'Herlihy C. Dystocia increases with advancing maternal age. Am J Obstet Gynecol. 2006;195(3):760–763. doi: 10.1016/j.ajog.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Wilkes PT, Wolf DM, Kronbach DW, Kunze M, Gibbs RS. Risk factors for cesarean delivery at presentation of nulliparous patients in labor. Obstet Gynecol. 2003;102(6):1352–1357. doi: 10.1016/j.obstetgynecol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organization technical report series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 22.Oshiro BT, Kowalewski L, Sappenfield W, Alter CC, Bettegowda VR, Russell R, et al. A multistate quality improvement program to decrease elective deliveries before 39 weeks of gestation. Obstet Gynecol. 2013;121(5):1025–1031. doi: 10.1097/AOG.0b013e31828ca096. [DOI] [PubMed] [Google Scholar]
- 23.Fisch JM, English D, Pedaline S, Brooks K, Simhan HN. Labor induction process improvement: a patient quality-of-care initiative. Obstet Gynecol. 2009;113(4):797–803. doi: 10.1097/AOG.0b013e31819c9e3d. [DOI] [PubMed] [Google Scholar]
- 24.Reisner DP, Wallin TK, Zingheim RW, Luthy DA. Reduction of elective inductions in a large community hospital. Am J Obstet Gynecol. 2009;200(6):674 e671–677. doi: 10.1016/j.ajog.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenthal DB, Jiang X, Strobino DM. Labor induction and the risk of a cesarean delivery among nulliparous women at term. Obstet Gynecol. 2010;116(1):35–42. doi: 10.1097/AOG.0b013e3181e10c5c. [DOI] [PubMed] [Google Scholar]
- 26.Darney BG, Snowden JM, Cheng YW, Jacob L, Nicholson JM, Kaimal A, et al. Elective induction of labor at term compared with expectant management: maternal and neonatal outcomes. Obstet Gynecol. 2013;122(4):761–769. doi: 10.1097/AOG.0b013e3182a6a4d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ. 2012;344:e2838. doi: 10.1136/bmj.e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Main EK, Moore D, Farrell B, Schimmel LD, Altman RJ, Abrahams C, et al. Is there a useful cesarean birth measure? Assessment of the nulliparous term singleton vertex cesarean birth rate as a tool for obstetric quality improvement. Am J Obstet Gynecol. 2006;194(6):1644–1651. doi: 10.1016/j.ajog.2006.03.013. discussion 1651-1642. [DOI] [PubMed] [Google Scholar]
- 29.Main EK, Bloomfield L, Hunt G. Development of a large-scale obstetric qualityimprovement program that focused on the nulliparous patient at term. Am J Obstet Gynecol. 2004;190(6):1747–1756. doi: 10.1016/j.ajog.2004.02.055. discussion 1756-1748. [DOI] [PubMed] [Google Scholar]
- 30.Spong CY. Preventing the First Cesarean Delivery Summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1181–1193. doi: 10.1097/aog.0b013e3182704880. [DOI] [PMC free article] [PubMed] [Google Scholar]


