Abstract
The ability of four 9,10-dihydroethanoanthracene derivatives (BG920, BG932, BG958, and BG996), as well as verapamil and promethazine, to reverse chloroquine resistance was assessed against 24 chloroquine-resistant and 10 chloroquine-susceptible strains of Plasmodium falciparum from different countries. The 9,10-dihydroethanoanthracene derivatives clearly increase chloroquine susceptibility only in chloroquine-resistant isolates.
The current option for reducing the morbidity and mortality of malaria are chemoprophylaxis and chemotherapy. Until recently, chloroquine (CQ) has been a key weapon in the fight against this disease. During the past 20 years, there has been an emergence of strains of Plasmodium falciparum resistant to CQ and other antimalarial drugs (27, 29). Failures of antimalarial prophylaxis with CQ, the combination of CQ and proguanil (4), and mefloquine (15, 23) and clinical failures with halofantrine (8) and quinine (S. Molinier, P. Imbert, D. Verrot, M. Morillon, D. Parzy, and J. E. Touze, Letter, Presse Med. 23:1484, 1994) have been observed in Africa. The spread of antimalarial drug-resistant strains increased the prevalence of malaria (29), which affects 40% of the world's population and kills more than 1 million people each year. One strategy that can be pursued to reduce the prevalence of malaria is to “reverse” CQ resistance chemically. In the past decade, several compounds, such as verapamil (VER) (1, 17, 24), desipramine (6, 7, 10-12), and antihistaminic drugs (5, 16, 19-21, 26), have demonstrated promising capability to reverse the CQ resistance in parasite isolates in vitro, in animal models, and in human malaria.
The aim of this study is to determine the capacity of four 9,10-dihydroethanoanthracenes (DEAs), BG920, BG932, BG958, and BG996 (Fig. 1), to reverse the resistance of several isolates of P. falciparum from different countries to CQ. These DEAs were selected from about 60 compounds. They exert more than 80% of in vitro reversal of CQ resistance at 1 μM (2). Their reversal activity 50% inhibitory concentrations (IC50s), i.e., the drug concentration of a modulator which induces an increase of 50% of the parasite susceptibility to CQ, are statistically better than those of VER or promethazine (PRM) (2). These chemosensitizers are less hemotoxic and cytotoxic in vitro than VER or PRM (2). In addition, it has been reported in previous studies that these DEAs increase the CQ accumulation in CQ-resistant (CQR) parasites 5 to 8 times more than PRM (2, 22).
FIG. 1.
Chemical structures of the reversing agents DEA derivatives, VER, PRM, and CQ.
Isolates of P. falciparum.
Thirty-four strains of Plasmodium falciparum, 27 isolates and 7 clones from several countries, were used for this study. Twenty-five isolates were African strains originating from nine countries, including Cameroon (two strains), Comoros (seven strains), Ivory Coast (four strains), Djibouti (two strains), Gabon (one strain), Gambia (one strain), Niger (two strains), Uganda (one strain), Senegal (five strains), and an undetermined African country (one strain). Six isolates came from three Asian countries, including Cambodia (four strains), Indochina (one strain), and Thailand (one strain). Additionally, one strain came from Brazil, and another was a Caribbean strain. Among the 34 parasites, 24 were CQR and 10 were CQ susceptible (CQS). All parasites were maintained in culture in RPMI 1640 medium (Invitrogen, Paisley, United Kingdom) supplemented with 10% human serum and buffered with 25 mM HEPES and 25 mM NaCO3 (hematocrit, 1.5%; parasitemia, 0.5%).
Drugs.
The synthesis of BG920, BG932, BG958, and BG996 was described previously (2, 3, 14). CQ, VER, and PRM were obtained from Sigma Chemical (St. Louis, Mo.). CQ was diluted in sterile distilled water. DEA derivatives, VER, and PRM were diluted in methanol. Final concentrations of CQ distributed for the evaluation of drug interaction ranged from 25 to 3,210 nM for resistant strains and from 3 to 200 nM for susceptible strains. VER, PRM, and DEAs were used at the following concentrations, corresponding to 1/5 and 1/20, respectively, of their intrinsic IC50s against the CQR clone W2: 2,600 and 650 nM for VER, 3,700 and 925 nM for PRM, 860 and 215 nM for BG920, 420 and 105 nM for BG932, 2,350 and 585 nM for BG958, and 1,600 and 400 nM for BG996. At these concentrations, none of the molecules has detectable antiplasmodial activity.
In vitro assay.
For in vitro isotopic microtests to assess synergy between CQ and DEA compounds, 25 μl of CQ, 25 μl of the drug tested, and 200 μl of the suspension of parasitized red blood cells (final parasitemia, 0.5%; final hematocrit, 1.5%) were distributed in 96-well plates. Parasite growth was assessed by adding 1 μCi of [3H]hypoxanthine with a specific activity of 14.1 Ci/mmol (Amersham, Buckinghamshire, United Kingdom) to each well at time zero. The plates were then incubated for 48 h at 37°C in an atmosphere of 10% O2, 5% CO2, and 85% N2, with a humidity of 95%. Immediately after incubation, the plates were frozen and then thawed to lyse erythrocytes. The contents of each well were collected on standard filter microplates (Unifilter GF/B; Perkin-Elmer, Courtaboeuf, France) and washed by using a cell harvester (FilterMate Cell Harvester; Perkin-Elmer). Filter microplates were dried, and 25 μl of scintillation cocktail (Microscint O; Perkin-Elmer) was placed in each well. Radioactivity incorporated by the parasites was measured with a scintillation counter (Top Count; Perkin-Elmer).
The IC50, i.e., the drug concentration corresponding to 50% of the uptake of [3H]hypoxanthine by the parasite in drug-free control wells, was determined by nonlinear regression analysis of log dose-response curves. Data were analyzed after logarithmic transformation and expressed as the geometric mean IC50.
Evaluation of drug effect.
To evaluate the capacities of the four DEAs, VER, and PRM to potentiate CQ activity against isolates of P. falciparum, an activity enhancement index of CQ was defined as the difference between the IC50 measured with CQ alone and the IC50 measured for CQ associated with molecules of potentiation divided by the IC50 measured for CQ alone.
Results.
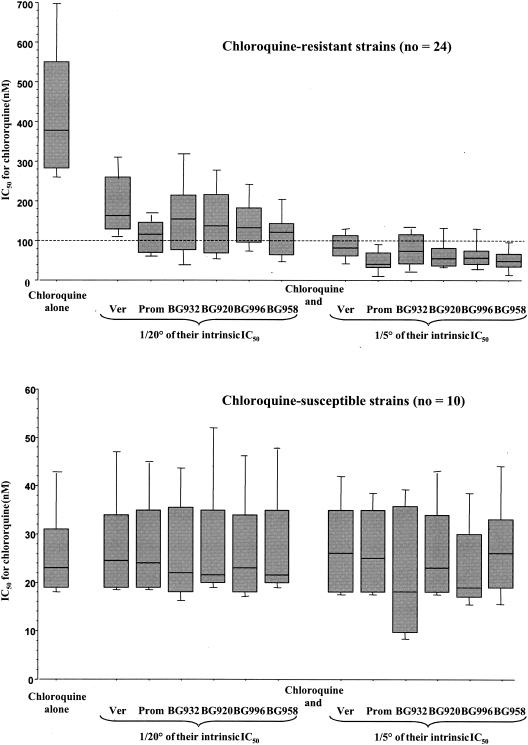
The CQ IC50 ranged between 156 and 858 nM for isolates resistant to CQ and between 17 and 65 nM for parasites susceptible to CQ (Fig. 2).
FIG. 2.
Box plots of mean IC50s (in nM) ± standard errors of the means of CQ alone or in combination with VER, PRM, BG920, BG932, BG958, and BG996 at two subinhibitory concentrations without detectable antiplasmodial activity, corresponding to 1/5 and 1/20 of their intrinsic IC50, against CQS and CQR isolates of P. falciparum.
The CQ IC50s against CQS isolates and clones were not significantly modified by combination with DEAs, VER, or PRM (Fig. 2). CQ IC50s against CQR isolates and clones were significantly reduced when combined with DEAs, VER, or PRM (P < 0.001). The CQ IC50s estimated in the presence of CQ were significantly higher than the CQ IC50s estimated in the presence of CQ associated with molecules of potentiation (P < 0.001).
The activity enhancement index of CQ by each molecule of potentiation at the studied concentration was significantly higher for CQR isolates compared to CQS parasites (P < 0,0001). Potentiation of CQ activity on CQR parasites is dose dependent (P < 0.001) (Fig. 2).
The activity enhancement index of CQ in the presence of BG958 was significantly higher than the indices measured in the presence of the other DEAs (P < 0,005). The increase of CQ activity in the presence of BG958 was equivalent to that observed in the presence of PRM for CQR isolates. BG958 at 585 and 2,350 nM reversed in vitro the CQ resistance, i.e., decreased CQ IC50 below the threshold of 100 nM, in 42% (10 out of 24) and 92% (22 out of 24) of CQR isolates, respectively (Table 1).
TABLE 1.
Reversion of CQ strains by combinations, of CQ and B6920, B6932, B6958, B6996 VER, or PRMa
| Drug with CQ (concnin μm) | Reversed strains for avg intrinsic IC50 fractions of:
|
|||
|---|---|---|---|---|
| 1/20
|
1/5
|
|||
| No. reversed/total isolates | % Reversed strains | No. reversed/total isolates | % Reversed strains | |
| B6920 (4.3) | 9/24 | 38 | 20/24 | 83 |
| B6932 (2.1) | 7/23 | 30 | 16/23 | 70 |
| B6958 (11.5) | 10/24 | 42 | 22/24 | 92 |
| B6996 (8) | 6/24 | 25 | 20/24 | 83 |
| VER (13) | 2/23 | 9 | 16/23 | 70 |
| PRM (18.5) | 8/23 | 35 | 23/23 | 100 |
For CQR strains, IC50 > 100 nm.
Discussion.
Quinoline resistance in P. falciparum is frequently compared to multidrug resistance in mammalian cells, in part because of the observations that CQ resistance could be reversed by P-glycoprotein substrates such as VER and PRM. Our results suggest that the mechanism of action of the DEAs is associated with resistance properties; the DEA reversal of resistance in P. falciparum is specific for resistant parasites. DEAs clearly increase CQ susceptibility in CQR isolates, although not to the levels of the naturally susceptible strains; this result has been reported with very few isolates (one to three isolates) for VER (9), desipramine (7, 12), and PRM (19). These DEAs fully or partially reversed resistance in all CQR isolates from all different geographic regions (Africa, Asia, and South America).
CQ resistance can be either fully or partially sensitive to the presence of VER, and recent reports suggest that this behavior may be associated with the presence of specific sequences in the pfcrt gene, which encodes a vacuolar transport protein (13). While genetic differences in CQ resistance are known (13, 18, 28), the reverse phenotypic response may exist in all geographic regions, independent of the allelic variations. However, it has been reported that VER reversibility was more pronounced in clones expressing recombinant pfcrt or strains from the Old World carrying the pfcrt CVIET haplotype than in parasite isolates or clones expressing the recombinant New World allele carrying the SVMNT haplotype (18, 25). It would be interest to know if these pfcrt polymorphisms were related to or were independent of the DEA CQ sensitization potential.
BG958 fully or partially reversed resistance in all CQR strains. The observation reported here suggests that BG958 is a good candidate for further studies. Evaluation of its in vivo reversal of CQ resistance and association with pfcrt and pfmdr1 polymorphisms is ongoing. Furthermore, the use of BG958 in combination with CQ, due to the low cost of BG958 synthesis, may be an economically viable proposition for developing countries and may be a more effective strategy than the introduction of another antimalarial drug at the national level.
Acknowledgments
This work is supported by the Délégation Générale pour l'Armement (03CO001 no. 010808/03-6). This work was carried out as a part of the COST B16 European Program.
REFERENCES
- 1.Adovelande, J., J. Deleze, and J. Schrevel. 1998. Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum. Biochem. Pharmacol. 55:433-440. [DOI] [PubMed] [Google Scholar]
- 2.Alibert, S., C. Santelli-Rouvier, B. Pradines, C. Houdoin, D. Parzy, J. Karolak-Wojciechowska, and J. Barbe. 2002. Synthesis and effects on chloroquine susceptibility in Plasmodium falciparum of a series of new dihydroanthracene derivatives. J. Med. Chem. 45:3195-3209. [DOI] [PubMed] [Google Scholar]
- 3.Alibert-Franco, S., C. Santelli-Rouvier, J. Barbe, B. Pradines, C. Houdoin, and D. Parzy. 1999. 9,10-(3′,4′-pyrrolidino)-9,10-dihydroanthracene and structurally related compounds as synergistic antimalarial drugs. Heterocyclic Comm. 5:235-240. [Google Scholar]
- 4.Barnes, A. J., E. L. Ong, E. M. Dunbar, B. K. Mandal, and E. G. L. Wilkins. 1991. Failure of chloroquine and proguanil prophylaxis in travellers to Kenya. Lancet 338:1338-1339. [DOI] [PubMed] [Google Scholar]
- 5.Basco, L. K., P. Ringwald, and J. Le Bras. 1991. Chloroquine potentiating action of antihistaminics in Plasmodium falciparum in vitro. Ann. Trop. Med. Parasitol. 85:223-228. [DOI] [PubMed] [Google Scholar]
- 6.Basco, L. K., and J. Le Bras. 1990. Desipramine or cyproheptadine for reversing chloroquine resistance. Lancet 335:422. [DOI] [PubMed] [Google Scholar]
- 7.Basco, L. K., and J. Le Bras. 1990. Reversal of chloroquine resistance with desipramine in isolates of Plasmodium falciparum from Central and West Africa. Trans. R. Soc. Trop. Med. Hyg. 84:479-481. [DOI] [PubMed] [Google Scholar]
- 8.Basco, L. K., J. Le Bras, C. Gillotin, P. Ringwald, E. Rabenjarson, F. Gimenez, O. Bouchaud, R. Farinotti, and J. P. Coulaud. 1991. Type RI resistance to halofantrine in West Africa. Trop. Med. Parasitol. 42:413-414. [PubMed] [Google Scholar]
- 9.Bayoumi, R. A., A. M. Creasey, H. A. Babiker, J. M. Carlton, A. A. Sultan, G. Satti, A. K. Sohal, D. Walliker, J. B. Jensen, and D. E. Arnot. 1993. Drug response and genetic characterization of Plasmodium falciparum clones recently isolated from a Sudanese village. Trans. R. Soc. Trop. Med. Hyg. 87:454-458. [DOI] [PubMed] [Google Scholar]
- 10.Bitonti, A. J., A. Sjoerdsma, P. P. McCann, D. E. Kyle, A. M. J. Oduola, R. N. Rossan, W. K. Milhous, and D. E. Davidson. 1998. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 242:1301-1303. [DOI] [PubMed] [Google Scholar]
- 11.Boulter, M. K., P. G. Bray, R. E. Howells, and S. A. Ward. 1993. The potential of desipramine to reverse chloroquine resistance of Plasmodium falciparum is reduced by its binding to plasma protein. Trans. R. Soc. Trop. Med. Hyg. 87:303. [DOI] [PubMed] [Google Scholar]
- 12.Carosi, G., S. Caligaris, G. Fadat, F. Castelli, A. Matteelli, D. Komka-Bemba, and G. Roscigno. 1991. Reversal of chloroquine resistance of wild isolates of Plasmodium falciparum by desipramine. Trans. R. Soc. Trop. Med. Hyg. 85:723-724. [DOI] [PubMed] [Google Scholar]
- 13.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. B. Ursos, A. B. Singh Sidhu, B. Naudé, K. W. Deitsch, X. Su, J. C. Wooton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frunzinski, A., J. Karolak-Wojciechowska, S. Alibert-Franco, C. Santelli-Rouvier, and J. Barbe. 1999. Synthesis and molecular structures of 11-benzylamido-9,10-dihydro-9,10-ethenoanthracenes. J. Chem. Crystallogr. 29:1201-1204. [Google Scholar]
- 15.Gari-Toussaint, M., B. Pradines, V. Mondain, A. Keundjian, P. Dellamonica, and Y. Le Fichoux. 2002. Senegal and malaria. True prophylactic failure of mefloquine. Presse Med. 31:1136. (In French.) [PubMed]
- 16.Kalkanidis, M., N. Klonis, L. Tilley, and L. W. Deady. 2002. Novel phenothiazine antimalarials: synthesis, antimalarial activity, and inhibition of the formation of β-hematin. Biochem. Pharmacol. 63:833-842. [DOI] [PubMed] [Google Scholar]
- 17.Martin, S. K., A. M. Oduola, and W. K. Milhous. 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 235:899-901. [DOI] [PubMed] [Google Scholar]
- 18.Mehlotra, R. K., H. Fujioka, P. D. Roepe, O. Janneh, L. M. B. Ursos, V. Jacobs-Lorena, D. T. McNamara, M. J. Bockarie, J. W. Kazura, D. E. Kyle, D. A. Fidock, and P. A. Zimmerman. 2001. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. USA 98:12689-12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oduola, A. M. J., A. Sowunmi, W. K. Milhous, T. G. Brewer, D. E. Kyle, L. Gerena, R. N. Rossan, L. A. Salako, and B. G. Schuster. 1998. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am. J. Trop. Med. Hyg. 58:625-629. [DOI] [PubMed] [Google Scholar]
- 20.Peters, W., B. L. Robinson, and L. B. Stewart. 2000. The chemotherapy of rodent malaria. LIX. Drug combinations to impede the selection of drug resistance, part 3: observations on cyproheptadine, an antihistaminic agent, with chloroquine. Ann. Trop. Med. Parasitol. 94:689-697. [DOI] [PubMed] [Google Scholar]
- 21.Peters, W., R. Ekong, B. L. Robinson, and D. C. Warhust. 1990. The chemotherapy of rodent malaria. XLV. Reversal of chloroquine resistance in rodent and human Plasmodium by antihistaminic agents. Ann. Trop. Med. Parasitol. 84:541-551. [DOI] [PubMed] [Google Scholar]
- 22.Pradines, B., S. Alibert, C. Houdoin, C. Santelli-Rouvier, J. Mosnier, T. Fusai, C. Rogier, J. Barbe, and D. Parzy. 2002. In vitro increase in chloroquine accumulation induced by dihydroethano- and ethenoanthracene derivatives in Plasmodium falciparum-parasitized erythrocytes. Antimicrob. Agents Chemother. 46:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringwald, P., S. Bartczak, J. Le Bras, F. Bricaire, S. Matheron, J. Bauchet, and J. P. Coulaud. 1990. Failure of anti-malarial prophylaxis with mefloquine in Africa. Trans. R. Soc. Trop. Med. Hyg. 84:348-349. [DOI] [PubMed] [Google Scholar]
- 24.Ryall, J. C. 1987. Reversal of chloroquine resistance in falciparum malaria. Parsitol. Today 3:256. [DOI] [PubMed] [Google Scholar]
- 25.Singh Sidhu, A. B., D. Verdier-Pinard, and D. A. Fidock. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowunmi, A., A. M. J. Oduola, O. A. T. Ogundahunsi, and L. A. Salako. 1998. Comparative efficacy of chloroquine plus chlorpheniramine and pyrimethamine/sulfadoxine in acute uncomplicated falciparum malaria in Nigerian children. Trans. R. Soc. Trop. Med. Hyg. 92:77-81. [DOI] [PubMed] [Google Scholar]
- 27.Trape, J. F., G. Pison, M. P. Preziosi, C. Enel, A. Desgrées du Loû, V. Delauney, B. Samb, E. Lagarde, J. F. Molez, and F. Simondon. 1998. Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. 321:689-697. [DOI] [PubMed] [Google Scholar]
- 28.Wellems, T. E., and C. V. Plowe. 2001. Chloroquine-resistant malaria. J. Infect. Dis. 184:770-776. [DOI] [PubMed] [Google Scholar]
- 29.Wernsdorfer, W. H. 1991. The development and spread of drug-resistant malaria. Parasitol. Today 7:297-303. [DOI] [PubMed] [Google Scholar]