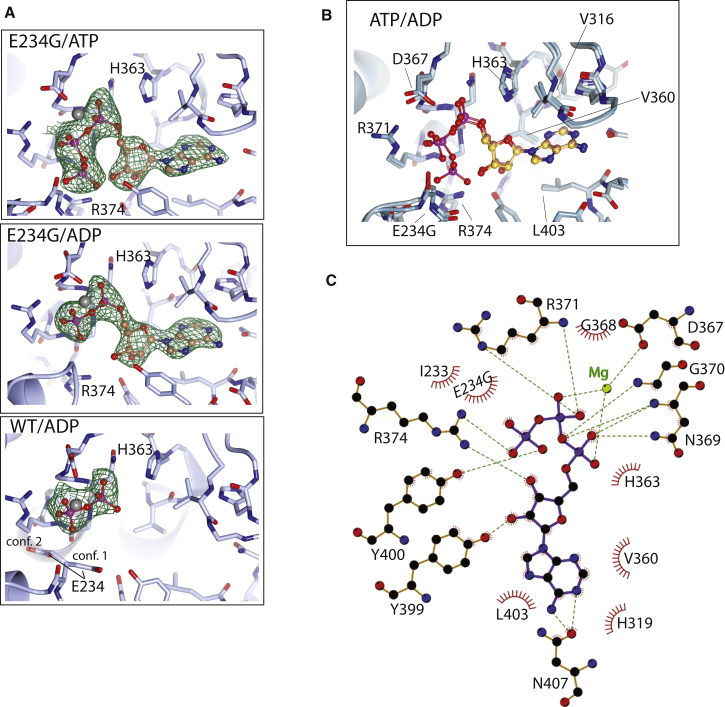
Figure 5.
Structures of HYPE Variants with Bound ADP and ATP
(A) Binding pocket for ATP and ADP in E234G and wild-type (WT) variants; density of the cofactors, catalytic His 363, and γ-phosphate coordinating Arg 374 are indicated. For WT/ADP, two conformations (more abundant conf. 1 and conf. 2) of the side chain Glu 234 are indicated. Electron density around the adenosine part of the ligand was poor, and so it is not shown for clarity.
(B) Overlay of ATP and ADP in the binding pocket of E234G variant; some of the key residues are labeled. See also Figure S6.
(C) Coordination of ATP by E234G variant, represented as a LigPlot+ diagram.