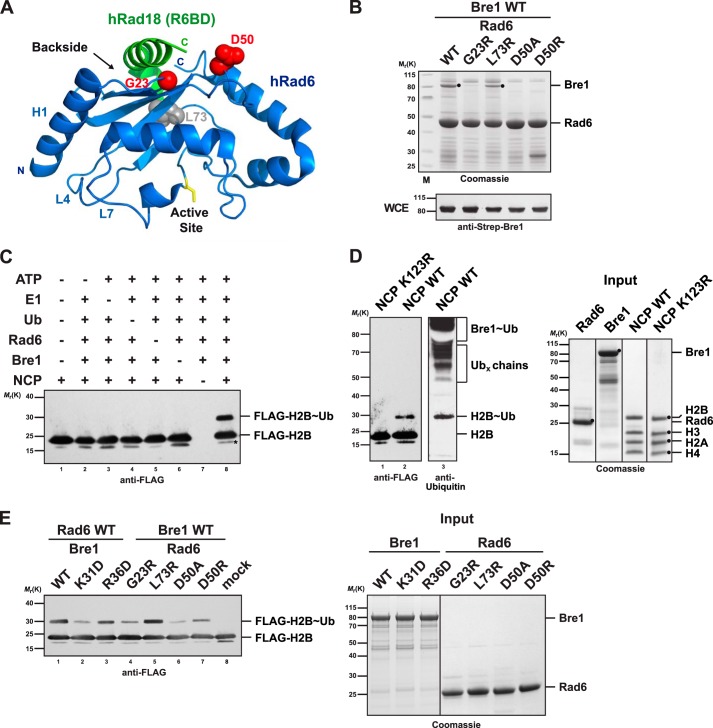
FIGURE 2.
Bre1 RBD binds to the Rad6 backside. A, ribbon structure of the human Rad6-Rad18 complex (Protein Data Bank 2YBF). Human and S. cerevisiae Rad6 are 85% identical and strictly conserved in the residues shown as spheres. Residues highlighted in red are involved in the Rad18 interaction (Asp-50) or are relevant for ubiquitin binding (Gly-23). The Rad6 backside and active site (yellow stick representation) are highlighted. The N-terminal helix1 (H1) and loops L4 and L7 comprise the canonical E1/E3-binding site. B, point mutations at the backside of Rad6 interfere with Bre1 binding. Strep-Bre1 and GST-Rad6 wild type (WT) or the indicated point mutants were co-expressed in E. coli and subjected to GST affinity purification. Proteins were eluted with GSH and analyzed by SDS-PAGE (4–12% gel, MES buffer) and Coomassie staining. Bre1 expression levels in E. coli whole cell extracts (WCE) were confirmed by immunoblotting with an anti-Strep antibody. C, requirements for the in vitro NCP ubiquitination reaction. The indicated combination of E1 (100 nm), Rad6 (3 μm), Bre1 (18 μm), ubiquitin (36 μm), and NCP (2 μm) was used. Reaction mixtures were analyzed by SDS-PAGE and immunoblotting against the FLAG-tagged histone H2B. Asterisk indicates a histone H2B degradation band. D, ubiquitination assay using the H2B K123R mutant NCP demonstrates site specificity of the reaction. Lanes 1 and 2 were probed with an anti-FLAG antibody and lane 3 with anti-ubiquitin antibody. Putative free Ub chains formed by Rad6 and bands corresponding to ubiquitinated Bre1 are indicated in the upper part of the gel in agreement with earlier reports (23, 28) Recombinant proteins used in the assays in C and D are shown in the Coomassie-stained gel on the right. E, Bre1 and Rad6 point mutants relevant for the E2 backside interaction show impaired NCP ubiquitination activity in vitro. NCP ubiquitination assay was performed with the indicated mutants. Input of the recombinant proteins is shown on the right.