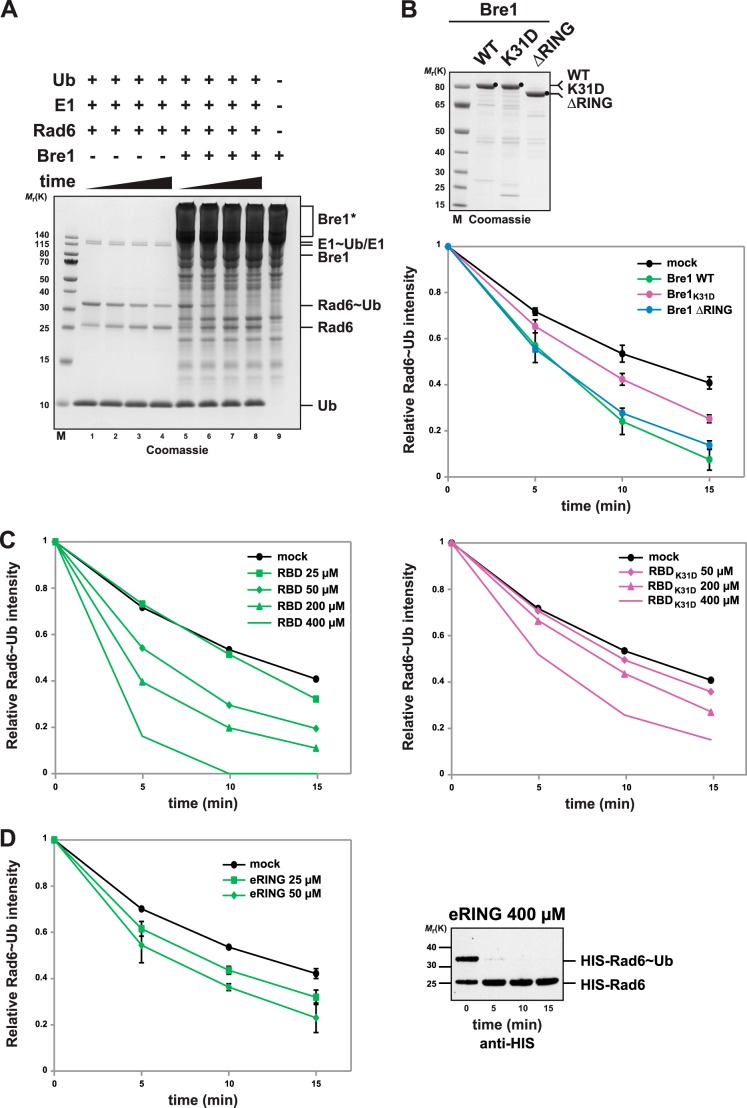
FIGURE 4.
Bre1 RBD is necessary and sufficient to promote Rad6 activation. A, Bre1 activates ubiquitin discharge from Rad6. Representative gel of a single turnover ubiquitin discharge experiment, in which Rad6, pre-charged with ubiquitin (Rad6∼Ub), was incubated without Bre1 (lanes 1–4) or with 25 μm Bre1 (lanes 5–8). Aliquots were collected after 0, 5, 10, and 15 min, and the reaction was stopped by denaturation. Samples were separated by nonreducing SDS-PAGE (12% gel, MES buffer) followed by Coomassie staining. Rad6 discharging is apparent by the time-dependent decrease of the Rad6∼Ub band intensity with a concomitant increase of the Rad6 band. Note that Rad6 can partially discharge without the E3. Lane 9 shows the Bre1 migration behavior under nonreducing conditions and formation of high molecular weight Bre1 aggregates marked by an asterisk. B, quantification of single turnover experiments as shown in A. Intensity of the Rad6∼Ub band was quantified from Coomassie-stained gels using ImageJ software, and the 0 time point was used for normalization. Three independent experiments per condition were used for quantification. Mean and standard deviation are indicated. Bre1 wild-type and mutant proteins used in the assay are shown on the Coomassie gel above. Mock indicates an assay without Bre1. C, single turnover assay showing Bre1-RBD stimulated Rad6 discharge and dose dependence of the reaction (left panel). Recombinant Bre1 RBD was added at the indicated concentrations. The same assay performed with the K31D mutant RBD (right panel) reveals critical role of RBD-Rad6 interaction for E2 activation. D, Bre1 eRING can activate Rad6. Rad6 discharging assay was performed with the indicated concentration of Bre1 eRING. Three independent experiments per condition were used for quantification. Mean and standard deviation are indicated. The eRING protein formed aggregates at high concentration (>100 μm) under nonreducing conditions, which increased the gel background and interfered with direct quantification of Rad6. To test Rad6 discharge at 400 μm eRING (right panel) His-Rad6 was detected by anti-His immunoblotting.