FIGURE 1.
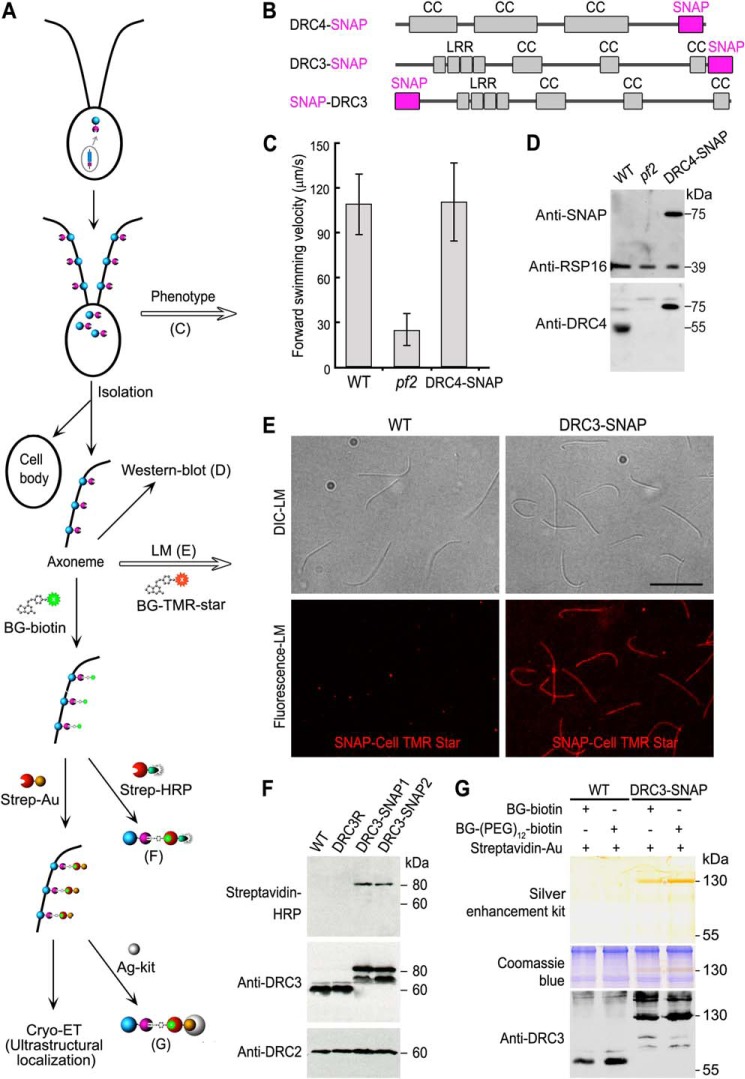
SNAP-tagged Chlamydomonas axonemal proteins assemble in the flagella, rescue the defects seen in the corresponding null mutants to WT phenotype, and can be ligated with BG-substrates with high specificity and efficiency. A, schematic workflow of the SNAP tag labeling technique for cryo-ET studies. B, diagrams showing predicted polypeptide domains in the SNAP-tagged DRC4 and DRC3, respectively. The SNAP tag is shown in magenta. Coiled coil domains (CC) and leucine-rich repeats (LRR) are shown in gray. C, measurements of forward swimming velocity indicate that the transformed DRC4-SNAP rescued the motility defects of the pf2 mutant. D, Western blots indicate that the transformed DRC4-SNAP assembled into the axoneme. Western blots of purified axonemes from WT, pf2, and DRC4-SNAP strains were probed with antibodies against SNAP, RSP16, and DRC4. The endogenous DRC4 protein (55 kDa) was only detected in WT axonemes, whereas a higher molecular mass band (75 kDa) corresponding to SNAP-tagged DRC4 protein was detected by both SNAP and DRC4 antibodies in the DRC4-SNAP rescued axonemes. The RSP16 antibody shows similar loading amounts of axonemal proteins. E, purified axonemes from WT (negative control) and DRC3-SNAP were probed with fluorescent SNAP-Cell® TMR-Star, a SNAP tag substrate (red), which showed the assembly of SNAP-tagged axonemal proteins along the full-length of Chlamydomonas axonemes. Scale bar, 10 μm. F, Western blots of purified axonemes from WT, DRC3R (a DRC3 rescue strain without a SNAP tag), and two DRC3-SNAP strains were probed with streptavidin-horseradish peroxidase (HRP) and with antibodies against DRC3 and DRC2, which showed that the SNAP tags in the rescue strains react with BG-biotin. An expected DRC3 (60 kDa) band was only detected in WT and DRC3R axonemes, and an expected DRC3-SNAP (80 kDa) band was only detected in DRC3-SNAP axonemes by both streptavidin-HRP and the anti-DRC3 antibody. This anti-DRC3 antibody also detected another band at 70 kDa in the DRC3-SNAP axonemes that did not respond to streptavidin-HRP. The DRC2 bands show similar loading amounts of axonemal proteins. G, SNAP-tagged DRC3 can be labeled with 1.4-nm-sized strep-Au with high specificity and efficiency. Purified axonemes from WT and DRC3-SNAP were labeled with the SNAP substrate BG-biotin or BG-(PEG)12-biotin, and then with strep-Au, which was visualized by silver enhancement kit (Ag-kit, top panel). A 130-kDa band was detected by silver enhancement (top) and in a duplicated gel by DRC3 antibodies (bottom panel) only in DRC3-SNAP axonemes. Post-staining of the same gel with Coomassie Blue (middle panel) confirmed equivalent loading amounts of axonemal proteins. Untagged DRC3 (60 kDa) was only detected in WT axonemes.