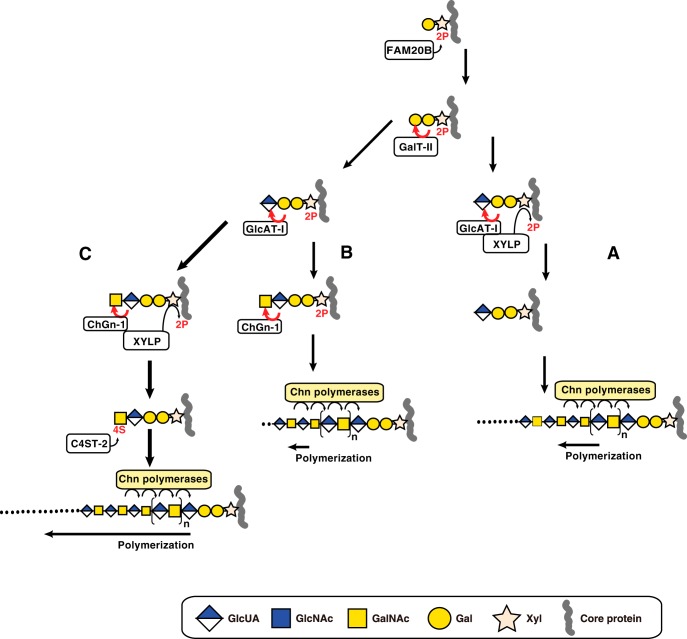
FIGURE 5.
Three different biosynthetic pathways for CS polymerization. Synthesis of the linkage region is initiated by the addition of a Xyl residue to a specific serine residue on the core protein followed by the sequential transfer of two Gal residues and is completed by transfer of a GlcUA residue. During synthesis of the linkage region, the Xyl residue is transiently phosphorylated by FAM20B, which enhances galactosyltransferase-II (GalT-II) and GlcAT-I activities. A, after synthesis of the phosphorylated linkage region trisaccharide, GlcAT-I transfers GlcUA to the phosphorylated trisaccharide Galβ1–3Galβ1–4Xyl(2-O-phosphate). Concomitantly, Xyl dephosphorylation is induced by XYLP. Chn polymerases then induce polymerization of the linkage region tetrasaccharide. B, following complete synthesis of the phosphorylated linkage region tetrasaccharide, ChGn-1 catalyzes the transfer of a single GalNAc residue to the phosphorylated tetrasaccharide linkage region. Then Chn polymerases may utilize the phosphorylated linkage region pentasaccharide albeit with low polymerization efficacy. C, following synthesis of the phosphorylated GalNAc linkage region, dephosphorylation is induced by XYLP. C4ST-2 subsequently mediates 4-O-sulfation of the non-reducing terminal GalNAc residue. Finally, the non-reducing terminal 4-O-sulfate of the GalNAc linkage structure facilitates elongation of the CS chains via Chn polymerases. 2P and 4S represent 2-O-phosphate and 4-O-sulfate, respectively. The arrow length of polymerization represents the efficiency of polymerization.