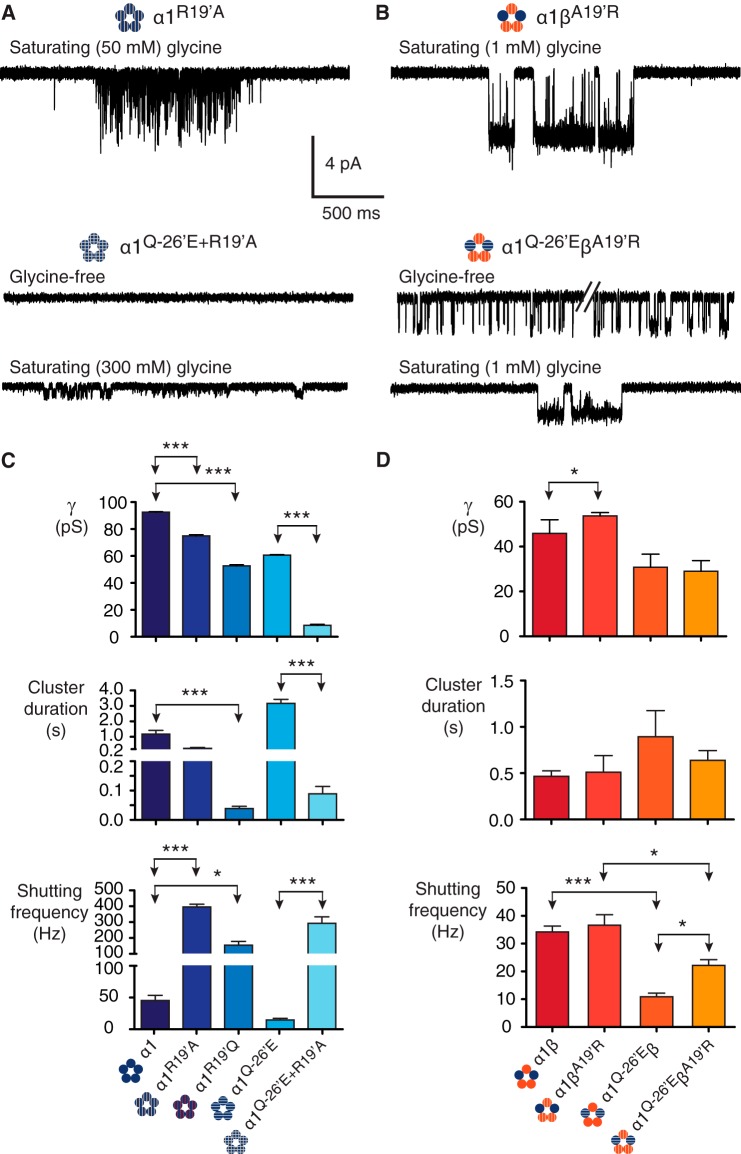
FIGURE 3.
Mutations to 19′. A, homomeric α1R19′A receptors show evidence of impaired receptor activation. When the α1R19′A mutation is combined with the α1Q−26′E mutation in the same subunit (α1R19′A/Q−26′E) and expressed as homomers, the functional impairment was greatest, ablating constitutive activity and markedly reducing conductance. B, heteromeric receptors containing the βA19′R subunit showed little evidence of functional impairment. When expressed with α1 wild-type (α1βA19′R) the receptors had similar properties to wild-type α1β heteromers. Similarly, when βA19′R was co-expressed with α1Q−26′E the resulting heteromers (α1Q−26′E-βA19′R) were little changed compared with the α1Q−26′E-β heteromers. C, group data summarizing the effects on channel conductance, cluster duration, and intra-cluster shutting frequency for receptors incorporating 19′ mutations. The number of clusters used in the analysis was 284 (α1R19′A, 4 patches), 378 (α1R19′Q, 5 patches), 99 (α1Q−26′E/R19′A, 10 patches), 35 (α1βA19′R, 3 patches), and 133 (α1Q−26′E-βA19′R, 5 patches). ***, p < 0.0001; *, p < 0.01.