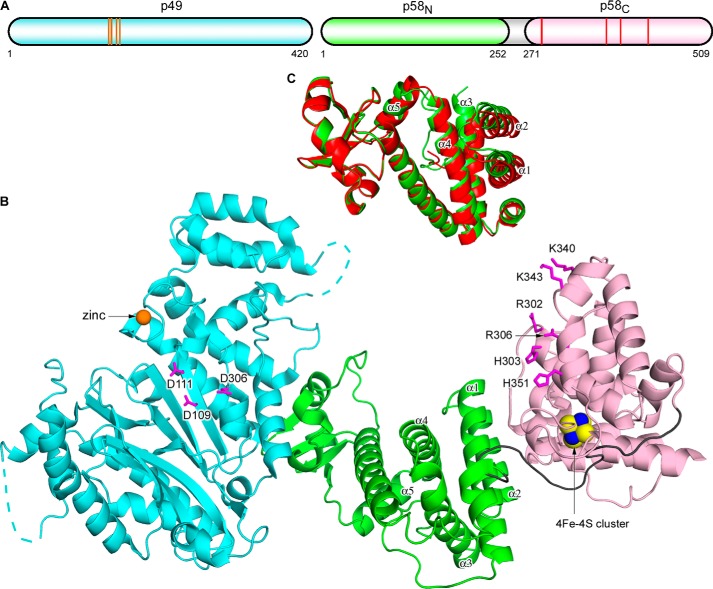
FIGURE 1.
Overall structure of the human primase. A, schematic representation of the domain organization. The orange lines and red lines in the schematics present relative positions of the residues coordinating zinc (Cys-121, Cys-122, Cys-128, and Cys-131) and the 4Fe-4S cluster (Cys-287, Cys-367, Cys-384, and Cys-424), respectively. B, schematic representation of p49-p58. The p49, p58N, p58C, and the linker between p58N and p58C are colored cyan, green, light pink, and gray, respectively. Zinc is shown as an orange sphere, and the 4Fe-4S cluster is shown as a space-filled representation, with iron and sulfur atoms colored blue and yellow, respectively. The disordered regions in p49 are shown by dashed lines. Side chains of catalytic aspartates on p49 and residues forming the proposed NTP/DNA binding site on p58C are shown as sticks and colored magenta. C, comparison of the two primase molecules in an asymmetric unit by superposition of p49. The p58N domain from one molecule (with p58C) is colored green, and that from a second molecule (without p58C) is colored red. p49 and p58C are omitted for clarity. The α-helices responsible for conformational flexibility of p58N are labeled.