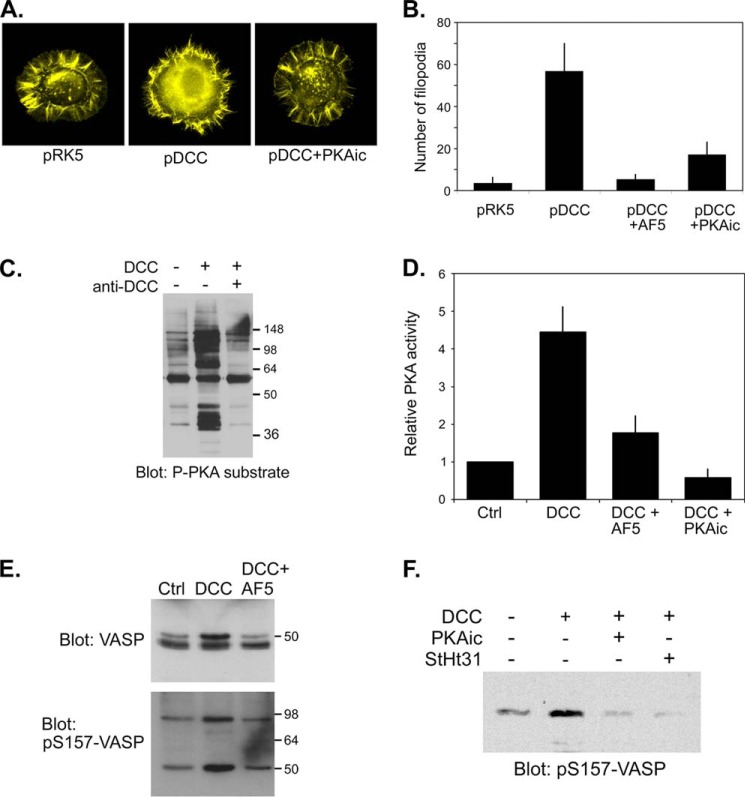
FIGURE 1.
Expression of DCC promotes filopodia formation and promotes activation of PKA and AKAP-dependent phosphorylation of Mena/VASP in NG108-15 neuroblastoma x glioma cells. A, NG108-15 cells were co-transfected with a plasmid encoding EGFP and either an empty plasmid (pRK5) or a plasmid encoding full-length rat DCC (pRK5-DCC) then replated onto polylysine-coated coverslips for 1 h in the absence or presence of a PKA inhibitor mixture (PKAic; see “Experimental Procedures”). Cells were fixed and stained with phalloidin and GFP-positive cells were visualized. B, NG108-15 cells were transfected as in A then replated in the absence or presence of the DCC-blocking AF5 antibody or PKA inhibitors (PKAic) onto polylysine-coated coverslips for 2 h. Cells were fixed and stained with phalloidin, and the number of filopodia per cell (n > 20 cells per condition) was measured. The bars represent the means and S.E from three different transfections. C, NG108-15 cells were transfected with empty pRK5 or with pRK5-DCC. Twenty-four hours later cells were replated onto polylysine/laminin-coated coverslips for 24 h in the absence (−) or presence of a function-blocking DCC antibody (anti-DCC; AF5). Whole cell extracts were analyzed by SDS-PAGE and immunoblotting using a phospho-PKA substrate antibody. D, NG108-15 cells were transfected with pRK5 (Ctrl) or pRK5-DCC and replated as above. For one sample, DCC-expressing cells plated in the absence of AF5 antibody were treated with PKA inhibitors (PKAic) for 30 min. PKA activity was assessed in an in vitro kinase assay. Data represent the means ± S.D. for three separate transfections. E and F, NG108-15 cells were transfected and cultured as in A. Where indicated, cells were replated in the presence of a DCC function-blocking antibody (AF5) or were treated for 30 min with PKA inhibitors (PKAic) or 50 μm concentrations of a cell-permeable peptide inhibitor of PKA anchoring (StHt31). Whole cell extracts were analyzed by immunoblotting with antibodies against total VASP or against the phosphorylated form of Ser-157 (pS157). The slower-migrating species in the total VASP blot corresponds to the Ser(P)-157 form. Note also that the Ser(P)-157 antibody cross-reacts with Mena at ∼95 kDa (41).