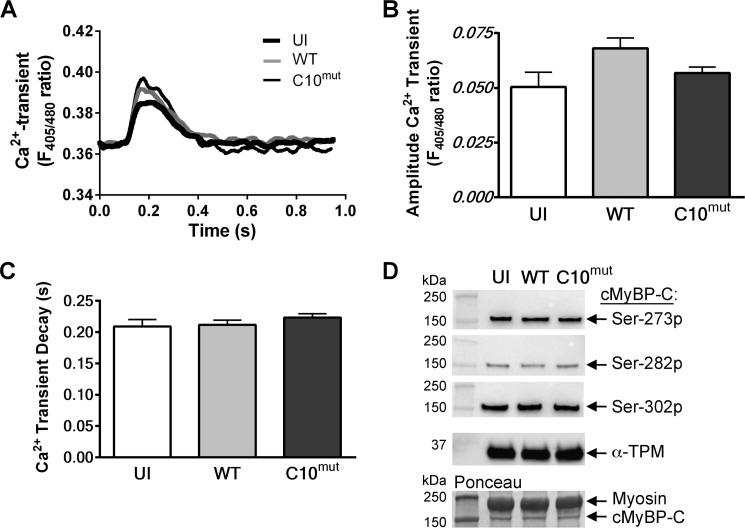
FIGURE 7.
Calcium transients and cMyBP-C phosphorylation levels in cultured cardiomyocytes. Concomitant with unloaded shortening, Ca2+ transients were measured. Cells were loaded with the Ca2+-sensitive fluorescent dye Indo-1. Ca2+ release into the cytosol was determined by measuring the ratio between Ca2+-bound Indo signal (at 405 nm) and Ca2+-unbound Indo signal (at 480 nm). A, representative traces of Ca2+ transients of uninfected (UI), cMyBP-CWT- and cMyBP-CC10mut-infected cells. B, no changes were seen in amplitude of the Ca2+ transient. C, Ca2+ reuptake kinetics (displayed as tau) was also unaffected by cMyBP-CC10mut expression. The data are means ± S.E. of four independent experiments. D, representative Western blot analyses of cMyBP-C phosphorylation at Ser-273 (Ser-273p), Ser-282 (Ser-282p), and Ser-302 (Ser-302p) sites using phospho-specific antibodies. cMyBP-C levels, as measured by Ponceau S staining, and α-TPM levels, as assessed by Western blot, were used as loading controls. 10 μg of total cultured cardiomyocyte lysate was used to perform the Western blot analyses. Quantitative data showed no significant difference among all the groups (data not shown).