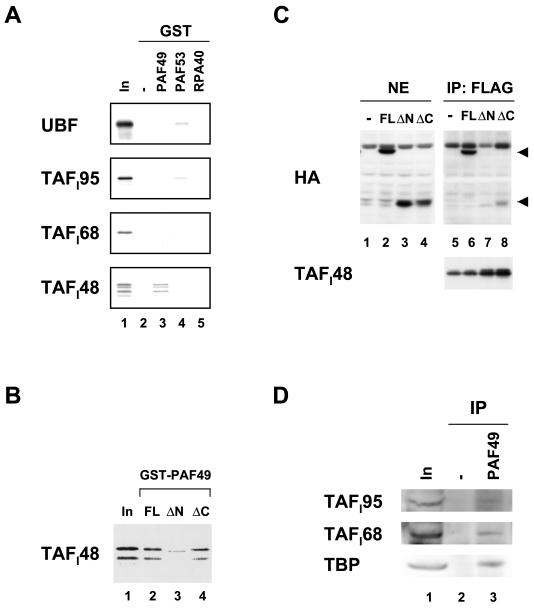
FIG. 6.
PAF49 interacts with TAFI48 in vitro and in vivo. (A) GST-PAF49 (lane 3), GST-PAF53 (lane 4), and GST-RPA40 (lane 5) as well as GST alone (lane 2) were immobilized on glutathione beads and then incubated with 35S-labeled UBF1 and the SL1 subunits TAFI95, TAFI68, and TAFI48. After extensive washing of the beads, bound proteins were eluted by boiling in SDS sample buffer and applied to an SDS-polyacrylamide gel. Ten percent of the input labeled protein was also included in lane 1. (B) GST-PAF49FL (lane 2), ΔN (lane 3), and ΔC (lane 4) were immobilized on glutathione beads and then incubated with 35S-labeled TAFI48. The bound protein was detected by autoradiography. Ten percent of the input labeled TAFI48 was also included (lane 1). (C) NIH 3T3 cells were cotransfected with plasmids expressing Flag-tagged TAFI48 and various forms of HA-tagged PAF49 as well as the empty vector. The expression of HA-tagged full-length PAF49 (FL, lane 2), the N-terminal truncation mutant (ΔN, lane 3), and the C-terminal truncation mutant (ΔC, lane 4) was confirmed in nuclear extracts by Western blotting. The extracts were then immunoprecipitated with anti-Flag M2 affinity beads (lanes 5 to 8). The positions of the full-length and C-terminally truncated forms of PAF49 in the immune complexes are indicated by arrowheads at the right. The amounts of Flag-tagged TAFI48 in the immune complexes are shown in the lower panel (lanes 5 to 8). (D) Nuclear extracts were incubated with (lane 3) or without (lane 2) anti-PAF49 antibodies, followed by precipitation with protein A beads. Each immune complex was analyzed by Western blotting with anti-TAFI95 antibody (upper panel), anti-TAFI68 antibody (middle panel), or anti-TBP antibody (lower panel). About 6% of the input nuclear extracts were also examined on the left (lane 1).