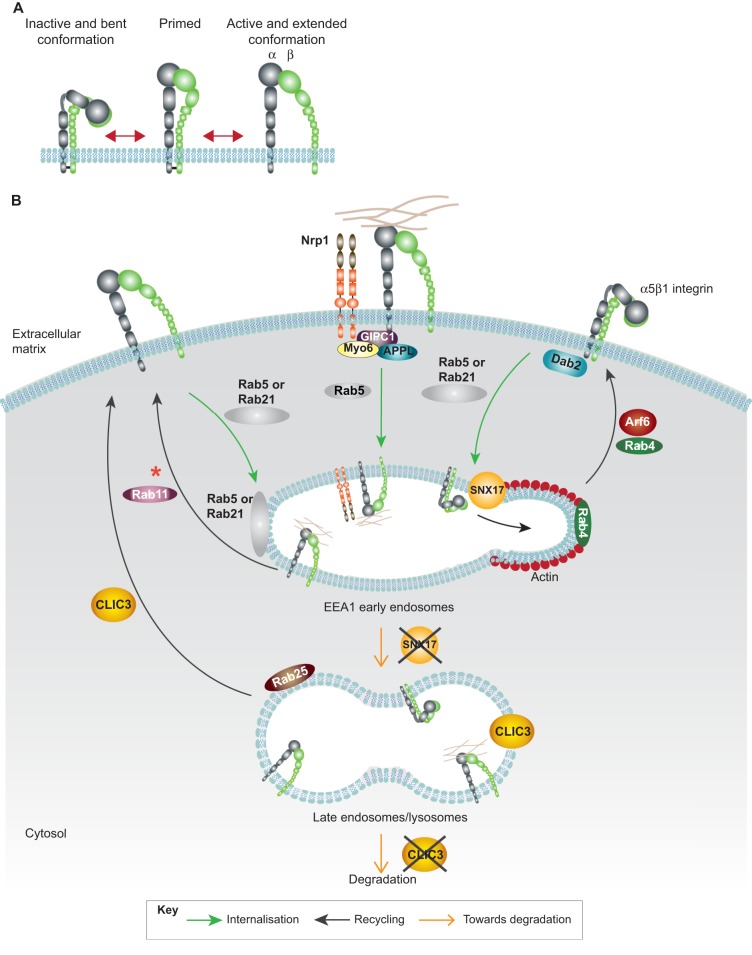
Fig. 2.
Trafficking of active and inactive integrin heterodimers. (A) Stepwise model of integrin activation through a conformational switch. (B) Schematic representation of the trafficking routes of active and inactive integrin heterodimers. Internalisation: at the plasma membrane, both active and inactive integrins are endocytosed to early endosomes in a Rab5- or Rab21-dependent fashion. Dab2 acts as an adaptor for endocytosis of inactive (unengaged) integrins, whereas an Nrp1–GIPC1–Myo6–APPL module mediates endocytosis of active α5β1 integrin from fibrillar adhesions. Recycling: inactive β1 integrins are rapidly recycled to Arf6-positive protrusions in a Rab4-dependent manner, whereas active receptors are trafficked through the Rab11 long-loop pathway (the red asterisk indicates recycling from PNRC – omitted here for simplicity). Degradation: in early endosomes, SNX17 binding to the cytoplasmic domain of inactive β-integrin promotes recycling of the receptor over degradation. Similarly, in the late endosome and lysosome compartment, CLIC3-mediated recycling prevents degradation of the active integrin receptor.