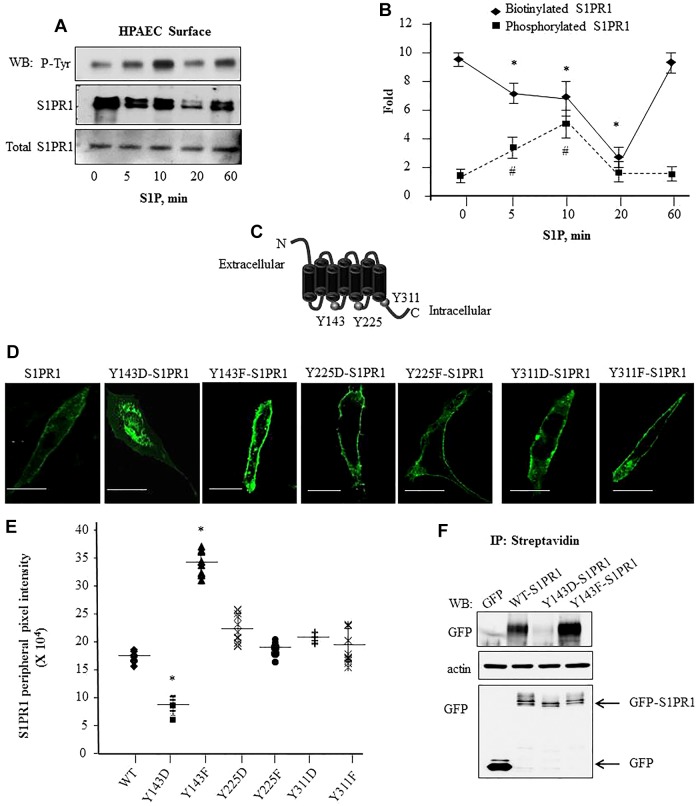
Fig. 2.
Phosphorylation of S1PR1 at Y143 is associated with reduced cell surface S1PR1 localization. (A,B) S1P-induced tyrosine phosphorylation is coupled to reduced cell surface retention of S1PR1. HPAECs were serum starved and stimulated with 1 µM S1P for the indicated times. Cell surface proteins were biotinylated followed by immunoprecipitation (IP) with anti-S1PR1 antibody; biotin-bound S1PR1 was isolated using streptavidin–agarose beads as indicated in the Materials and Methods. Protein lysates were separated by SDS-PAGE and immunoblotted (WB) with anti-phosphotyrosine (P-Tyr) or anti-S1PR1 antibodies. Total cell lysates were assessed for S1PR1 to confirm equal protein loading. A shows a representative immunoblot blot and B shows the mean±s.d. fold change in phosphorylation or biotinylated S1PR1 versus total S1PR1 expression at each time. Fold increase was calculated taking values at ‘0’ time as 1. from multiple experiments. *P<0.05 for a reduction compared to at 0 min; #P<0.05 for an increase in compared to at 0 min. (C) Schematic showing potential tyrosine residues in S1PR1 that can be phosphorylated. (D,E) Phosphorylation at Y143 reduces GFP–S1PR1 cell surface localization. (D) CHO cells were transfected with the indicated mutants and after 24 h cells were visualized using confocal microscopy. Scale bars: 10 µm. (E) The mean pixel intensity (line) at the cell periphery from various cells was quantified as described in the Materials and Methods. Plot shows pixel intensity at the cell periphery in cells expressing the mutants from three independent experiments. In each experiment at least ten cells were counted. *P<0.05 compared with cells expressing WT-S1PR1. (F) Cell surface proteins in CHO cells transiently transfected with indicated GFP-tagged constructs were biotinylated as described in the Materials and Methods. Biotinylated proteins were immunoprecipitated with anti-GFP antibody to detect receptor cell surface expression. Lysates were immunoblotted with anti-GFP antibody to assess S1PR1 expression. Immunoblotting with anti-β-actin antibody was performed to assess equal protein loading control. A representative blot from three experiments performed independently is shown.