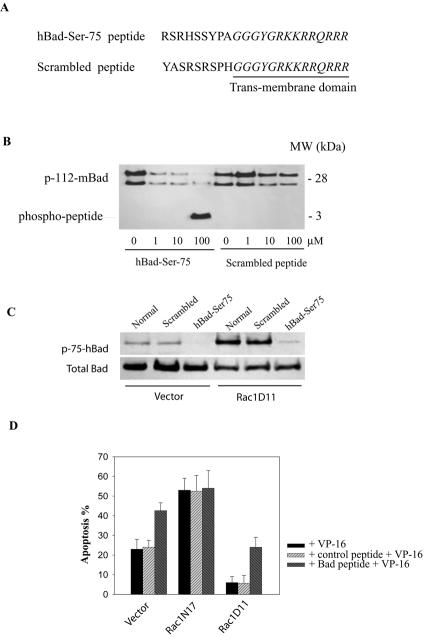
FIG. 3.
A competitive inhibitor of hBad Ser-75 phosphorylation sensitizes lymphoma cells to VP-16-induced apoptosis. (A) Sequence of cell-permeable hBad-Ser-75 peptide and the control (scrambled) peptide. The hBad-Ser-75 peptide contains the serine-75 phosphorylation site (amino acids 70 to 78) of hBad. The PTD (underlined) derived from the HIV-1 Tat protein promotes cell entry. (B) Effects of hBad Ser-75 peptide on Bad phosphorylation in vitro. Recombinant murine Bad (mBad; 0.5 μg) was incubated in standard PKA assay buffer with active PKAC (20 ng) and increasing amounts (1 to 100 μM) of peptides in a final volume of 70 μl for 10 min at 30°C. The reactions were stopped by addition of SDS sample buffer and were analyzed for Bad phosphorylation by Western blotting with anti-pSer-112-mBad antibodies. (C) Effects of peptides on Bad phosphorylation in vivo. Cells were treated with 500 μM hBad-Ser75 or scrambled peptide for 2 h and were analyzed for the status of Bad phosphorylation on Ser-75. (D) Effect of hBad Ser-75 peptide on the sensitivity of lymphoma cells to VP-16-induced apoptosis. Cells were pretreated with 500 μM peptide for 1 h and then were incubated with VP-16 (200 μg/ml) for an additional 2.5 h. Apoptosis was assessed by annexin V-fluorescence-activated cell sorting analysis. The results represent the means ± standard deviations from three independent experiments. MW, molecular size.