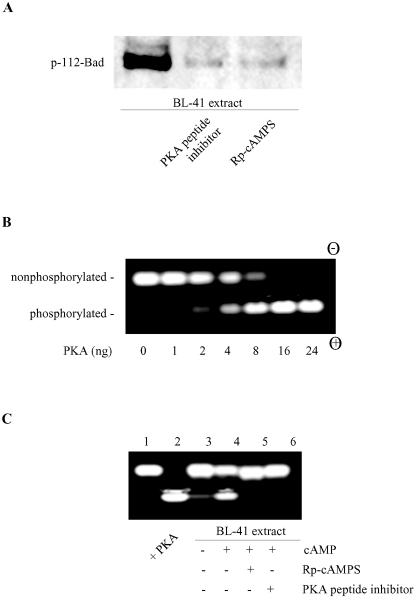
FIG. 5.
Inhibition of cellular PKA activity blocks Bad phosphorylation. (A) BL-41 cell extracts (200 ng of total protein) were assayed for Bad kinase activity in the absence or presence of a PKA peptide inhibitor (20 μM) or Rp-cAMPS (100 μM). (B) PepTag assay for determining PKA activity. PepTag A1 peptide (2 μg), a PKA-specific substrate, was incubated in standard PKA assay buffer with various amounts (1 to 24 ng) of active PKAC in a final volume of 25 μl for 30 min at room temperature. The reactions were stopped and then subjected to agarose gel electrophoresis. The phosphorylated peptide substrate migrates toward the positive electrode (+), while the nonphosphorylated peptide migrates toward the negative electrode (−). (C) Detection of PKA-specific kinase activity in cell lysates. Extracts (2 μg) from healthy BL-41 cells were subjected to the PepTag PKA assay as described for panel B in the absence (lane 3) or presence (lanes 4 to 6) of cAMP. The PKA peptide inhibitor (20 μM) or Rp-cAMPS (100 μM) was added where indicated (lanes 5 and 6). Lanes 1 and 2 show the negative (buffer only) and positive controls (with 10 ng purified PKAC). The data represent one of two independent experiments.