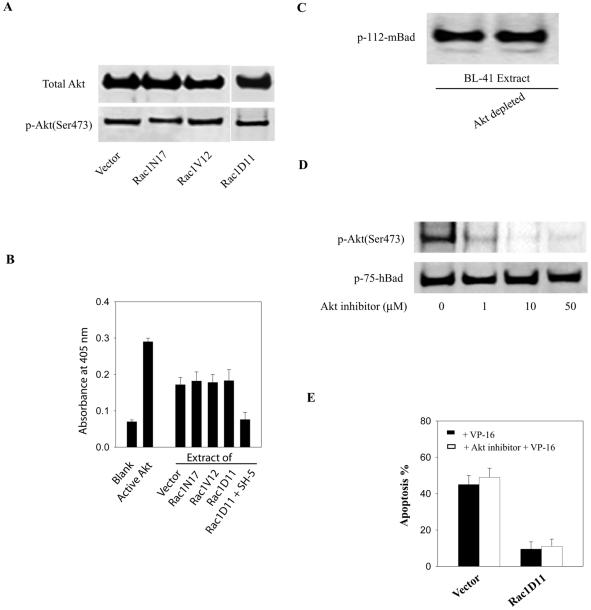
FIG. 7.
Akt activity is not responsive to Rac1 activity. (A) The activation state of Akt in stable lymphoma cells expressing Rac1 mutants was assessed by measuring the level of Akt phosphorylation by using an antibody against phospho-Akt (Ser-473). Equal loading was assessed by reprobing the membrane with an anti-Akt antibody. (B) Akt-specific kinase activities were measured by a colorimetric Akt kinase assay as described in Materials and Methods. The kinase reactions were carried out with equal amounts of cell extracts from the indicated cell lines. The positive control contains purified recombinant Akt. Inhibition of Akt activity in cell extracts was accomplished with the Akt-specific inhibitor SH-5. (C) Phosphorylation of Bad by whole (left lane) or Akt-depleted (right lane) cell extracts was assessed as described in the legend to Fig. 1. (D) Stable Rac1D11-expressing cell lines were treated with increasing amounts of Akt inhibitor (SH-5) for 1 h. Whole-cell lysates were analyzed by Western blotting with the indicated phosphospecific antibodies to detect Akt phosphorylation and activation and Bad phosphorylation. (E) Effect of the Akt inhibitor on the sensitivity of the lymphoma cells to VP-16-induced apoptosis. Cells were pretreated with 10 μM Akt inhibitor (SH-5) for 1 h and then were incubated with VP-16 (200 g/ml) for an additional 3 h. Apoptosis was assessed by the annexin V-fluorescence-activated cell sorting assay. The results represent the means ± standard deviations from three independent experiments.