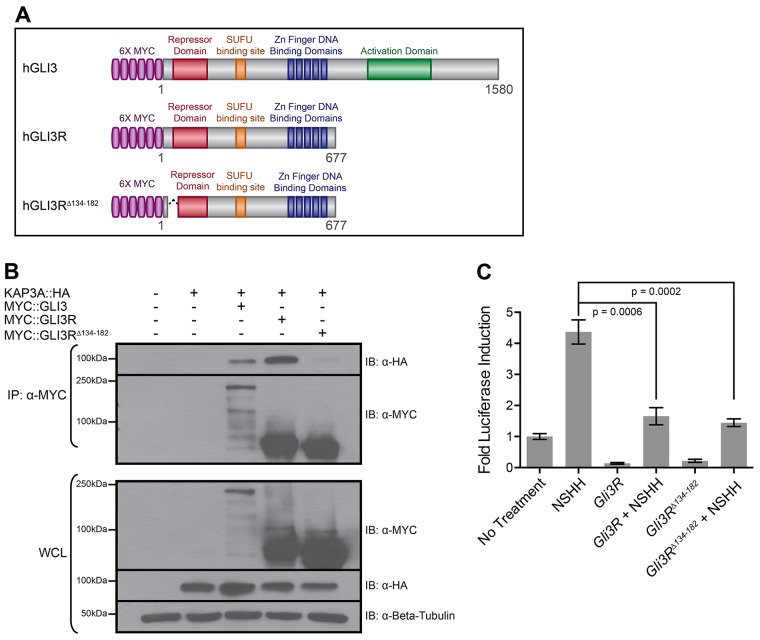
Fig. 6.
KAP3A interacts with an N-terminal GLI3 domain that does not alter GLI repressor function. (A) Schematic of full-length and truncated human (h)GLI3 proteins. (B) Immunoprecipitation (IP) of MYC-tagged GLI3 (MYC::GLI3), GLI3R (MYC::GLI3R) or GLI3RΔ134–182 (MYC::GLI3RΔ134–182) from COS-7 cells expressing HA-tagged KAP3A (KAP3A::HA). Immunoprecipitates (IP) and whole-cell lysates (WCL) were subjected to SDS-PAGE and western blot analysis (IB) using antibodies directed against MYC (α-MYC) and HA (α-HA). Antibody detection of β-tubulin (α-Beta-Tubulin) was used to confirm equal loading across lanes. (C) Luciferase activity readout of HH signaling after transfection with empty vector (No Treatment), Gli3R or Gli3Δ134–182. HH pathway activity is measured as the fold luciferase induction. Data represent the mean±s.d. for triplicate samples in a single experiment and are representative of three independent experiments; P-values are indicated above the relevant treatment groups (Student's unpaired t-test).