Figure 4.
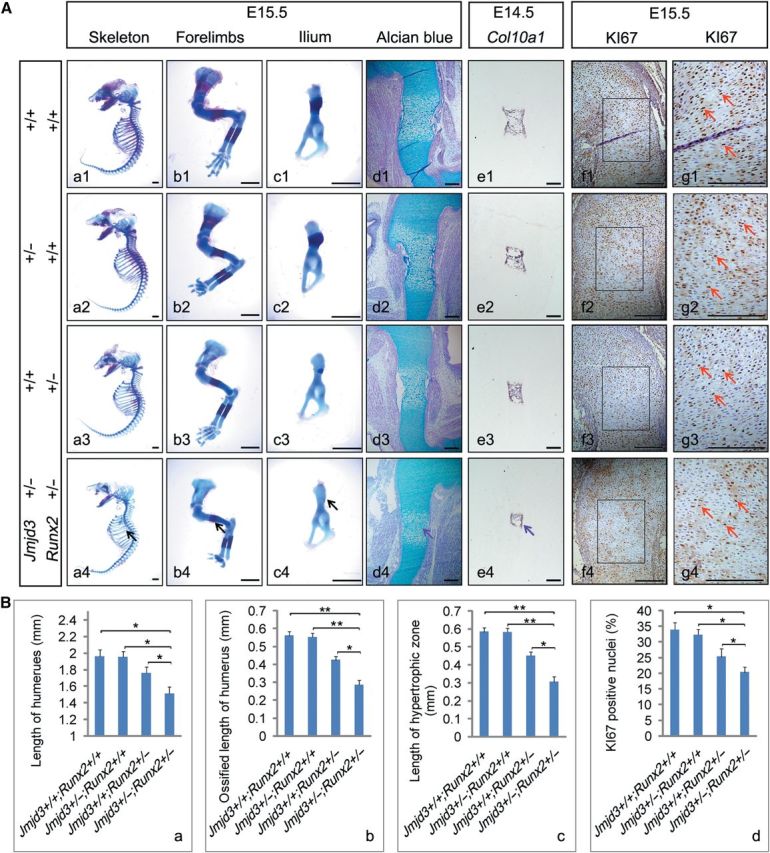
JMJD3 genetically cooperates with RUNX2 to regulate chondrocyte proliferation and hypertrophy during endochondral ossification. (A) Representative skeletal preparation (a1–c4), alcian blue staining (d1–d4), in situ hybridization with Col10a1 (e1–e4) probe, and immunohistochemistry of KI67 counterstained with hematoxylin (f1–f4) of Jmjd3+/+; Runx2+/+ (first row), Jmjd3+/–; Runx2+/+ (second row), Jmjd3+/+; Runx2+/– (third row), and Jmjd3+/–; Runx2+/– (bottom row) mice at E15.5 (a1–d4; f1–g4) and E14.5 (e1–e4). The boxed regions in f1–f4 are magnified in g1–g4, respectively. Black arrows indicate the most delayed bone ossification in ribs (a1–a4), forelimbs (b1–b4), and ilium (c1–c4) of Jmjd3+/–;Runx2+/– mice. Purple arrows indicate the shortest hypertrophic zone of chondrocytes in humeri of Jmjd3+/–;Runx2+/– mice at E15.5 (d4) and E14.5 (e4). Red arrows indicate KI67-positive nuclei (g1–g4). Scale bar, 1 mm in a1–c4 and 200 μm in d1–g4. (B) Quantification of full humerus length (a), length of ossified zone of humeri (b), length of hypertrophic zone of humeri (c), and proliferation rate in cartilage growth plate represented by the percentage of KI67-positive cells (d) of Jmjd3+/+; Runx2+/+, Jmjd3+/–; Runx2+/+, Jmjd3+/+; Runx2+/–, and Jmjd3+/–; Runx2+/– mice at E15.5. *P < 0.05, **P < 0.01. Two-tailed Student's t-test. Error bar represents the SE (n = 3).
