Abstract
Objective
To review clinical and structural outcomes of ocriplasmin for stage 2 macular holes.
Methods
A retrospective IRB approved review of the first patients with stage 2 macular holes to receive ocriplasmin at Massachusetts Eye and Ear Infirmary. All patients were imaged with spectral domain optical coherence tomography (SD-OCT).
Results
Eight patients with stage 2 macular holes, as defined by vitreomacular adhesion with narrowest hole diameter <400 μm, received a single injection of 125 μg of ocriplasmin. Only one patient (12.5%) demonstrated macular hole closure. The posterior hyaloid separated from the macula in 6 (75%) eyes. In those seven holes that remained open, all showed enlargement of their hole diameters (narrowest, apical, and basal) at one week and one month. All 7 eyes that failed to close with ocriplasmin closed with surgery. Ellipsoid zone disruptions were observed by OCT in 4 (50%) eyes and persisted throughout the follow-up. The average total follow-up was over 6 months.
Conclusions
In our early clinical results, we found a lower macular hole closure rate with ocriplasmin than previously reported. We also observed macular hole enlargement in all patients that failed to close with ocriplasmin. Using SD-OCT, we identified ellipsoid zone disruptions that persisted through six months of follow-up after ocriplasmin injection. Further work is needed to investigate the cause for these EZ changes.
Introduction
No successful treatment options for macular hole existed until 1991, when Kelly and Wendell reported the use of vitrectomy. 1 Although modifications have been introduced to their original technique, its basic principles have remained the standard of care for full-thickness macular holes for the past two decades. However, the risks of vitrectomy, such as cataract and retinal detachment, and the need for face-down positioning, has fueled continued interest in non-surgical intervention.
Given the role of vitreoretinal adhesion in macular hole formation, 2 investigators have searched for pharmacologic agents capable of vitreolysis. 3 Plasmin is a serine protease with enzymatic activity against two key components of the vitreous scaffold, laminin and fibronectin. Both plasmin and its catalytic substrate, microplasmin (ocriplasmin), have been shown to be effective vitreolytics in both animal models and early human studies. 4-8
A phase I clinical trial established safety and phase II and III trials showed ocriplasmin to be both safe and effective in relieving vitreomacular adhesion. 9-11 A subgroup of the phase 3 study involved 106 eyes with stage 2 macular holes. Successful hole closure occurred in 40.6% of the ocriplasmin group compared with 10.6% of controls.
We reviewed our early clinical experience in a consecutive series of eyes that received ocriplasmin shortly after the drug became commercially available. Furthermore, by using spectral domain optical coherence tomography (OCT) (Heidelberg Spectralis®) instead of the time-domain OCT used in the phase 3 trial, we are able to provide new information regarding structural changes to the retina following ocriplasmin injection.
Methods
We reviewed the charts of the first eight patients who received a single intravitreal injection of ocriplasmin 125 micrograms for stage 2 macular hole at the Massachusetts Eye and Ear Infirmary. Presenting clinical findings, intervention, and follow-up findings were recorded. All patients were examined using spectral domain optical coherence tomography (OCT) (Heidelberg Spectralis®). OCTs were reviewed for vitreoretinal interface abnormalities, macular hole features, and retinal architecture. Institutional review board approval was obtained prior to conducting this review.
Results
The average age of the patients was 67.5 years old. Six patients were female and two were male. 6 eyes were phakic and 2 were pseudophakic. The median visual acuity on presentation was logMAR 0.7 (Snellen 20/100). All eyes had stage 2 macular holes, as defined by OCT with confirmed vitreomacular adhesion and narrowest diameter less than 400 μm. The average presenting narrowest diameter was 162.25 μm, with average apical and basal diameters of 238 μm and 664 μm, respectively. Six holes were classified as small, narrowest diameter <250 μm, and two were medium, narrowest diameter 250-400 μm. None of these holes were associated with an observable epiretinal membrane on OCT. Of the 6 patients with documented refractive error, none of the patients had myopia > -8 diopters. The average follow-up was 189 days after ocriplasmin injection with a range of 77-258 days. Seven of the eight patients had follow-up greater than 166 days or nearly 6 months.
One week after ocriplasmin injection, only one of the eight macular holes closed (Figure 1). The other seven macular holes were still open at one week and one month (Figure 2). Visual acuity in the one hole that closed decreased to 0.6 (20/80) at one week despite hole closure from 0.5 (20/63) at presentation, but then improved to 0.2 (20/30) by the one month follow-up. In those that failed to close, the mean visual acuities first decreased from 0.90 (∼20/159) at presentation to 0.98 (20/192) at one week, before slightly improving to 0.92 (∼20/165) at one month. After the injection, two patients complained of severe photopsias, one had yellowing of vision, and two reported a two day episode of colorful moving images. Five patients noted new floaters after the injection.
Figure 1.
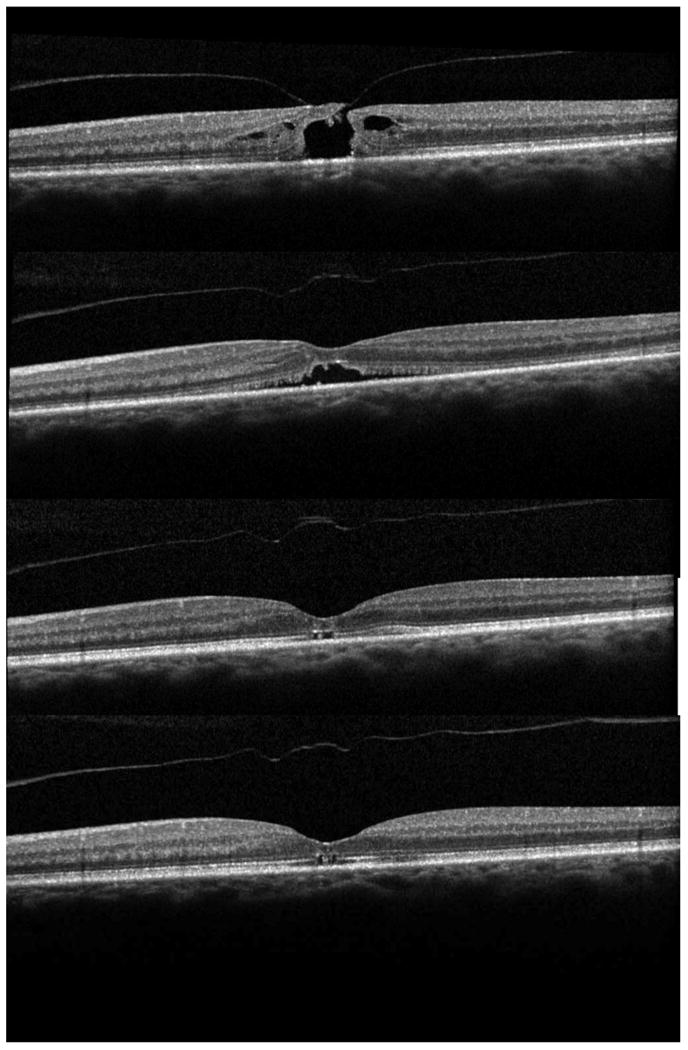
Successful closure with ocriplasmin. A: Stage 2 macular hole. B: One week after ocriplasmin injection with separation of the posterior hyaloid and closure of the inner retina. C: One month after ocriplasmin injection with hole closure and outer retinal defects. D: Six months after ocriplasmin injection with persistent outer retinal defects and ellipsoid zone (EZ) disruptions.
Figure 2.
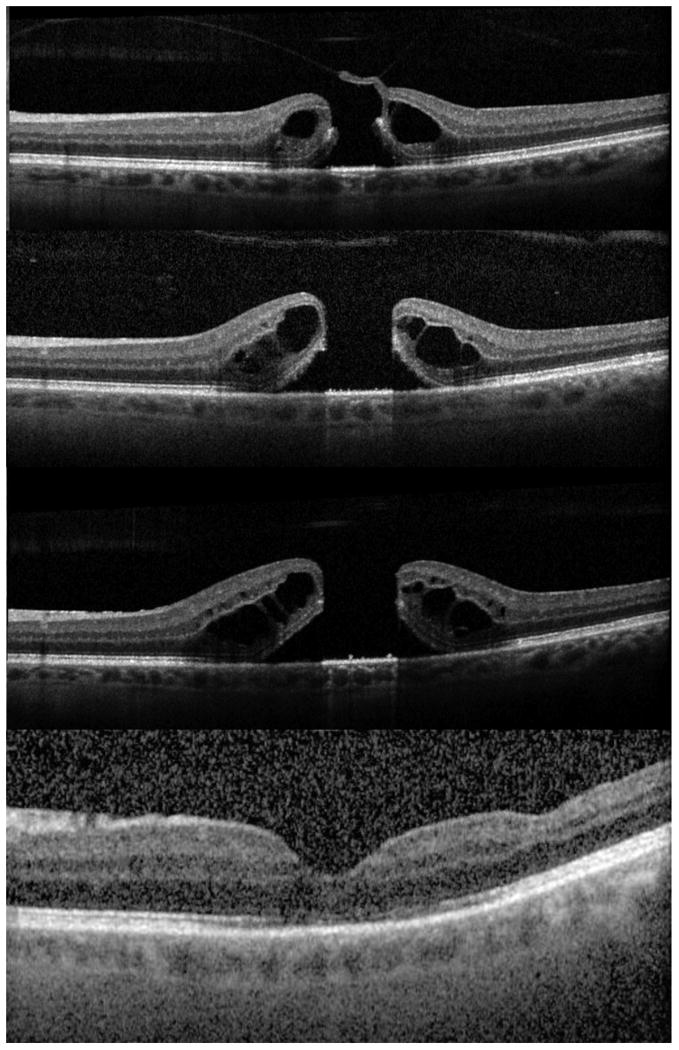
Ocriplasmin failure A: Stage 2 macular hole prior to treatment. B: One week after ocriplasmin injection showing posterior vitreous detachment and enlargement of the macular hole. C: One month after ocriplasmin injection with further progression of intraretinal cystic changes and macular hole diameters. D: Seven months after surgical repair showing macular hole closure.
Changes in hole dimensions by OCT
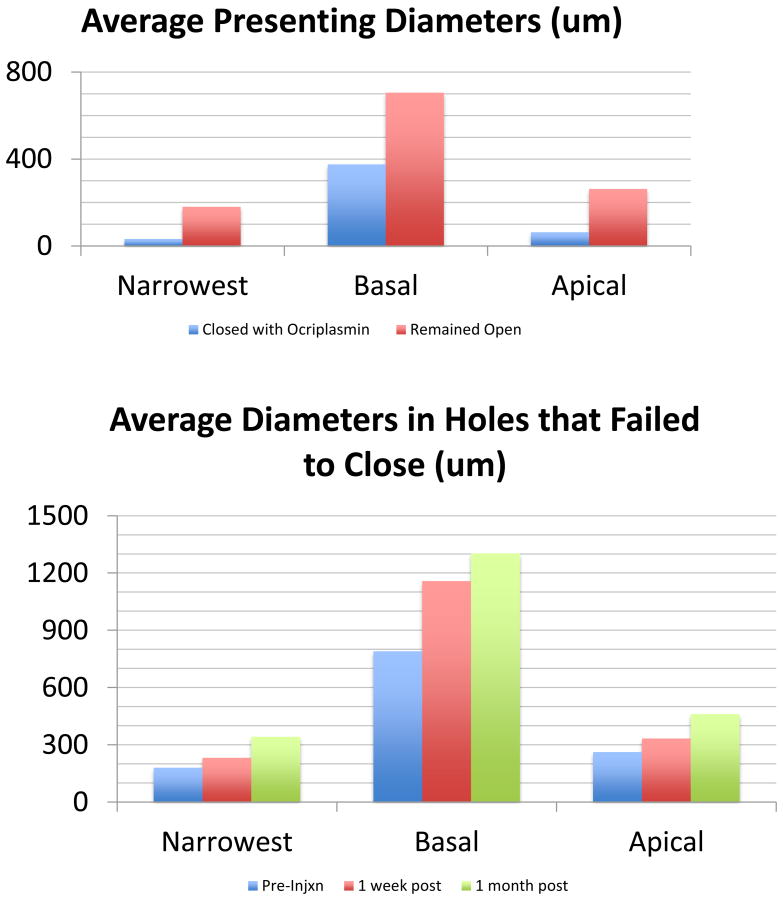
In comparing initial OCT measurements, the one hole that closed was considerably smaller than those that failed. The narrowest (N), apical (A), and basal (B) initial diameters of the hole that closed were 32 μm, 63 μm, and 375 μm, respectively as compared to the average diameters of 181 μm (N), 263 μm (A), and 705 μm (B) in those that failed (Figure 3A).
Figure 3.
OCT measurements A: The average diameters in the one hole that closed with ocriplasmin were smaller than those that failed to close. B: The average diameters of those holes that failed to close with ocriplasmin showed enlargement from pre-injection to 1 week post-injection to 1 month post-injection. These were statistically significant changes (*) in basal diameter at one week (p=0.002) and one month (p=0.006) and apical diameter at one month (p=0.016).
In those that remained open after ocriplasmin, all seven holes showed enlargement of all three diameters at one week. In the six eyes with OCTs at one month, all three measurements showed further enlargement one month after the injection. The average increase in diameters in those that failed was 52 μm (N), 72 μm (A), and 452 μm (B) at one week and 162 μm (N), 199 μm (A), and 598 μm (B) at one month (Figure 3B). These were statistically significant changes from presentation in basal diameter at both one week (p=0.002) and one month (p=0.006) and apical diameter at one month (p=0.016). The most obvious change by OCT was an increase in subretinal fluid from a mean basal diameter of 705 μm at presentation to 1158 um at one week and 1303 μm at one month.
Posterior hyaloid changes with Ocriplasmin
The posterior hyaloid fully separated in four eyes one week after ocriplasmin injection, including the one hole that closed. One eye demonstrated vitreous separation over the macula but not at the optic nerve. In the other three eyes, the posterior hyaloid remained identical to its pre-injection appearance at one week. At one month after injection, one additional eye developed complete vitreous separation. In total, six of eight eyes developed separation of the posterior hyaloid from the macula after ocriplasmin by one month. Despite the release of the vitreous, only one macular hole closed.
Surgical closure
All seven eyes that failed to close underwent vitrectomy, posterior hyaloid removal, and gas tamponade. Six eyes underwent internal limiting membrane (ILM) peeling, with four of these assisted by ICG staining. The average time to surgery after injection was 66 days, with a range of 23-105 days. Macular hole closure occurred in all 7 eyes after a single vitrectomy. The mean final visual acuity in those undergoing surgical repair improved to logMAR 0.54 (∼ 20/69) at the last visit compared to logMAR 0.91 (∼20/165) pre-operatively.
Ellipsoid zone changes after Ocriplasmin
One week after ocriplasmin injection, four of the eight eyes had changes in the ellipsoid zone (EZ). Specifically, we noted attenuation of the EZ band and focal defects in the otherwise linear EZ band. These changes persisted throughout all follow-ups scans (Figure 4).
Figure 4.
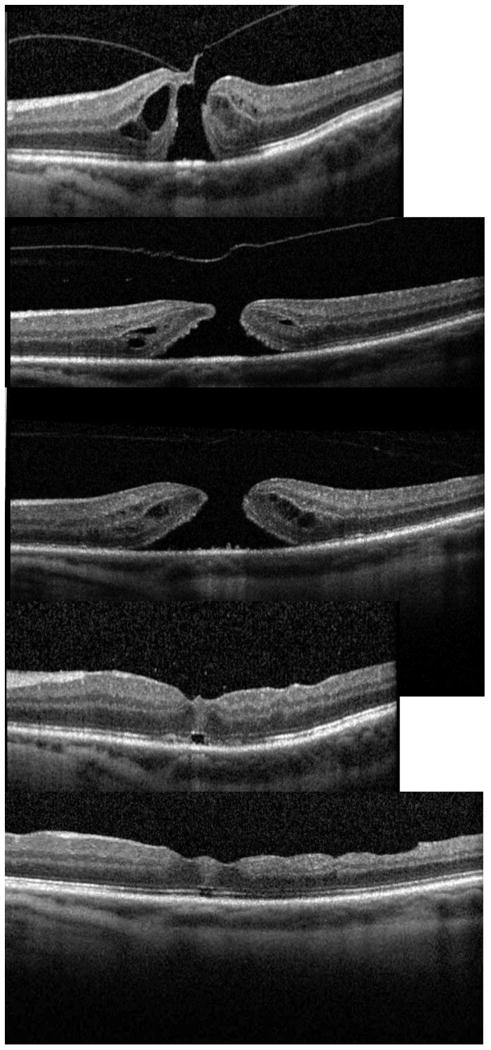
EZ attenuation. A: Stage 2 macular hole prior to treatment. B: One week after ocriplasmin injection showing loss of the EZ nasally and attenuation of the EZ temporally. C: One month after ocriplasmin injection showing continued attenuation of the EZ nasally and temporally. D: Four months after surgery with continued subfoveal outer retinal defect and attenuation of EZ band, but some improvement in the EZ from prior OCT.
In those holes that underwent surgical repair, we noted additional EZ changes in the post-operative scans of four eyes that had no previous EZ abnormalities. Again, these changes included attenuation of the EZ band and focal defects of the EZ band. These changes persisted through the last follow-up.
Discussion
Ocriplasmin is an important new therapeutic tool for non-surgical management of symptomatic vitreomacular adhesion (VMA). An intravitreal injection of ocriplasmin is currently indicated for vitreomacular adhesion resulting in stage 2 macular hole <400 μm in diameter. Stalmans et. al reported a closure rate of 40.6% of ocriplasmin treated eyes compared with 10.6% of sham eyes.11 Since the phase 3 trial, there have been two published reports of early clinical outcomes for ocriplasmin in VMA.12,13 In subgroup analysis, they reported macular hole closure in 3 of 6 patients12 and 4 of 5 patients13 after ocriplasmin. We review our own experience with intravitreal ocriplasmin exclusively for stage 2 macular holes. With longer follow-up and spectral domain OCT, we are able to provide new information about structural changes in the retina after ocriplasmin.
Our review found a closure rate of 12.5% at 1 week and 1 month after ocriplasmin injection. In the eye with the macular hole that closed, the hole size was small, particularly the apical diameter and narrowest diameter. In those holes that failed to close, OCT revealed that all eyes had enlargement of all diameters after injection at one week, with further progression at one month. This may represent the natural progression of these stage 2 holes. It also offers an early prognostic sign, given the low likelihood for non-surgical closure if hole enlargement is observed. The high rate of closure with vitrectomy, low risk of complications, and cost of procedure need to be considered when offering treatment options to patients with stage 2 macular hole.
Using SD-OCT, Freund et. al first reported loss of the ellipsoid zone in a patient one week after receiving ocriplasmin for VMA.14 They reported partial recovery of the EZ by OCT at one month, suggesting a transient drug toxicity to the photoreceptors. In our series, we observed EZ disruptions by OCT in half of our patients after ocriplasmin injection, at both the one week and one month exams. In addition, our patients still showed EZ disturbances after an average follow-up of six months. The greater duration of these changes could be explained to some extent by the additional surgical intervention that the ocriplasmin failures received. However, there were also persistent changes in our case of successful pharmacologic closure 174 days after ocriplasmin alone, suggesting a sustained effect with ocriplasmin. Our findings may represent a longer lasting potential drug toxicity to the photoreceptors. We hypothesize that this could in part be due to increased access of ocriplasmin to the outer retina, particularly the photoreceptors, through the full thickness retinal defect of a macular hole. Whether the observed EZ disturbances are related to drug mediated toxicity or a direct mechanical effect on the outer retina has not yet been determined. The cause of this persistent disruption of the EZ needs to be further investigated in a larger study, with the use of SD-OCT or swept source OCT.
Ocriplasmin has been shown to be useful in releasing vitreomacular adhesion. However, revised patient selection criteria for its use in macular holes may be needed. Duker et. al recently published a new classification system for the vitreomacular interface, including macular holes.15 Their efforts augment Gass' clinical definitions of macular hole by incorporating OCT findings into their classification. Specifically, they divide stage 2 macular holes into the subcategories small <250 μm and medium 250-400 μm and quantify the amount of vitreomacular adhesion (VMA). A more refined classification by OCT may better identify candidates with a higher chance for successful closure with ocriplasmin.
We report a lower closure rate after ocriplasmin in patients with stage 2 macular holes compared with the clinical trial results. Our OCT scans identified an increase in hole diameters in all eyes that failed to close with ocriplasmin. We also noted multiple cases with EZ disruptions after ocriplasmin. Most importantly, these changes persisted through 6 months after injection. Further work is needed to investigate this potential toxicity to photoreceptors in a larger series with more advanced OCT technology. A more narrowly defined inclusion criteria of macular holes for ocriplasmin treatment may lead to improved success rates. At the very least, physicians may need to temper patient expectations for hole closure after ocriplasmin.
Footnotes
Financial Support: None.
The authors have no conflicts of interest.
This manuscript has not been previously presented.
References
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Gass J. Stereoscopic atlas of macular diseases: diagnosis and treatment. 1987 [Google Scholar]
- 3.Rhéaume MA, Vavvas D. Pharmacologic vitreolysis. Semin Ophthalmol. 2010;25:295–302. doi: 10.3109/08820538.2010.518865. [DOI] [PubMed] [Google Scholar]
- 4.Verstraeten TC, Chapman C, Hartzer M. Pharmacologic induction of posterior vitreous detachment in the rabbit. Archives of …. 1993 doi: 10.1001/archopht.1993.01090060139038. [DOI] [PubMed] [Google Scholar]
- 5.Takano A, et al. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am J Ophthalmol. 2005;140:654–660. doi: 10.1016/j.ajo.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Uemura A, et al. Effect of plasmin on laminin and fibronectin during plasmin-assisted vitrectomy. Arch Ophthalmol. 2005;123:209–213. doi: 10.1001/archopht.123.2.209. [DOI] [PubMed] [Google Scholar]
- 7.Gandorfer A. Microplasmin-Assisted Vitrectomy. Pharmacology and Vitreoretinal Surgery. 2009;44:26–30. doi: 10.1159/000223942. [DOI] [PubMed] [Google Scholar]
- 8.Gandorfer A. Posterior Vitreous Detachment Induced by Microplasmin. Investigative Ophthalmology & Visual Science. 2004;45:641–647. doi: 10.1167/iovs.03-0930. [DOI] [PubMed] [Google Scholar]
- 9.de Smet MD, et al. Microplasmin Intravitreal Administration in Patients with Vitreomacular Traction Scheduled for Vitrectomy. Ophthalmology. 2009;116:1349–1355.e2. doi: 10.1016/j.ophtha.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Stalmans P, Delaey C, de Smet MD, van Dijkman E, Pakola S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial) Retina (Philadelphia, Pa) 2010;30:1122–1127. doi: 10.1097/IAE.0b013e3181e0970a. [DOI] [PubMed] [Google Scholar]
- 11.Stalmans P, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367:606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 12.Kim BT, et al. Initial outcomes following intravitreal ocriplasmin for treatment of symptomatic vitreomacular adhesion. Ophthalmic Surg Lasers Imaging Retina. 2013;44:334–343. doi: 10.3928/23258160-20130715-05. [DOI] [PubMed] [Google Scholar]
- 13.Singh RP, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. British Journal of Ophthalmology. 2014;98:356–360. doi: 10.1136/bjophthalmol-2013-304219. [DOI] [PubMed] [Google Scholar]
- 14.Freund KB, Shah SA, Shah VP. Eye_Freund. Eye. 2013;27:773–774. doi: 10.1038/eye.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MD, D JS, et al. The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. Ophthalmology. 2013;120:2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]