Abstract
In patients with a language developmental delay, it is necessary to make a differential diagnosis for autism spectrum disorders (ASDs), specific language impairment, and mental retardation. It is important that pediatricians recognize the signs and symptoms of ASDs, as many patients with language developmental delays are ultimately diagnosed with ASDs. Pediatricians play an important role in the early recognition of ASDs, because they are usually the first point of contact for children with ASDs. A revision of the diagnostic criteria of ASDs was proposed in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) that was released in May 2013. The autism spectrum describes a range of conditions classified as neurodevelopmental disorders in the fifth edition of the DSM. The new diagnostic criteria encompasses previous elements from the diagnosis of autistic disorder, Asperger disorder, childhood disintegrative disorder, and pervasive developmental disorder-not otherwise specified. An additional change to the DSM includes synthesizing the section on social and communication deficits into one domain. In ASD patients, the appropriate behavioral therapies and rehabilitation treatments significantly affect the prognosis. Therefore, this makes early diagnosis and treatment very important. In conclusion, pediatricians need to be able to recognize the signs and symptoms of ASDs and be attentive to them in order to make an early diagnosis and provide treatment.
Keywords: Autism spectrum disorder, Developmental disabilities, Genetics
Introduction
In Korea, the national health screening program for infants and children (zero to six years of age) has been implemented since November 2007, to monitor the growth and development of infants and children as well as to provide parents with appropriate education programs. For children aged 9 months, 18 months, 30 months, and 5 years, the Korean-Ages and Stages Questionnaire (K-ASQ) and the Denver-II developmental assessments are used. When infants or children show questionable scores, they are referred to professional institutions for a confirmatory diagnosis of developmental delay. According to the report on the effects of the health screening for infants and children, developmental delays were suspected in 0.6%-2.5% of the children tested and 99% of these children were diagnosed with neurodevelopmental disorders. The final distribution of diagnoses included global developmental delay (50%), developmental language disorder (26%), cerebral palsy (10%), motor developmental delay (9%), and autistic disorders (4%)1).
Among the developmental disorders, developmental language disorder was the second most common; in addition, many autism spectrum disorders (ASDs) were reported. In language delay patients, it is necessary to make a differential diagnoses for ASDs, specific language impairment, and mental retardation. It is important that pediatricians recognize the signs and symptoms of ASDs as many patients with language developmental delays are ultimately diagnosed with ASDs. Pediatricians play an important role in the early recognition of ASDs, because they are usually the first point of contact for patients.
The ASDs represent a wide continuum of associated cognitive and neurobehavioral deficits, including deficits in socialization and communication, with restricted and repetitive patterns of behavior. ASDs are organic neurodevelopmental disorders caused by genetic or neurobiological factors rather than by psychological or environmental ones. Recently, many studies on the genetics of ASDs have been conducted. In the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), published in May 2013, the diagnostic criteria for ASDs have subsequently been revised.
In this article, the most recent diagnostic criteria and the latest research on genetic studies with ASDs have been reviewed.
Historical background
Kanner2) first documented a syndrome of "autistic disturbances" in 11 children who shared previously unreported patterns of behavior, including poor social interaction, obsessiveness, stereotypic movement, and echolalia. Autism, the prototypic pervasive developmental disorder (PDD), is characterized by an onset prior to 3 years of age and by a triad of behavioral signs and symptoms, including (1) abnormal development in the use of language; (2) lack of reciprocal social interaction and responsiveness; and (3) restricted, stereotypical, and ritualized patterns of interests and behavior3).
The Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; 1994) and the DSM-IV-TR (text revision; 2000) included five possible diagnoses under the PDD: autistic disorder, Asperger disorder, childhood disintegrative disorder, Rett syndrome, and PDD-not otherwise specified (NOS). A revision of the ASDs was proposed in the DSM-5, released in May 20134). The autism spectrum describes a range of conditions classified as neurodevelopmental disorders in the fifth edition of the DSM. The new diagnosis encompasses the previous diagnostic criteria for autistic disorder, Asperger syndrome, childhood disintegrative disorder, and PDD-NOS.
It is believed that individuals with ASDs are best represented as a single diagnostic category because they show similar types of symptoms and are better differentiated by clinical specifiers (i.e., dimensions of severity) and associated features (i.e., known genetic disorders, epilepsy, and intellectual disability). An additional change to the DSM-5 includes synthesizing the social and communication deficits section into one domain.
Diagnostic criteria
The diagnostic criteria of autism in the DSM-IV consisted of three domains: social interaction impairment, communication deficits, and stereotypic behavior. These were condensed into two domains in the DSM-5: deficits in social communication and restricted patterns of behavior (Table 1). Additionally, the autism categories in the DSM-IV included autistic disorder, Asperger disorder, Rett syndrome, childhood disintegrative disorder, and PDD-NOS. However, these were converted to only one category in the DSM-5: ASD, with various severity levels added (Table 2).
Table 1. DSM-5 diagnostic criteria for autism spectrum disorders.
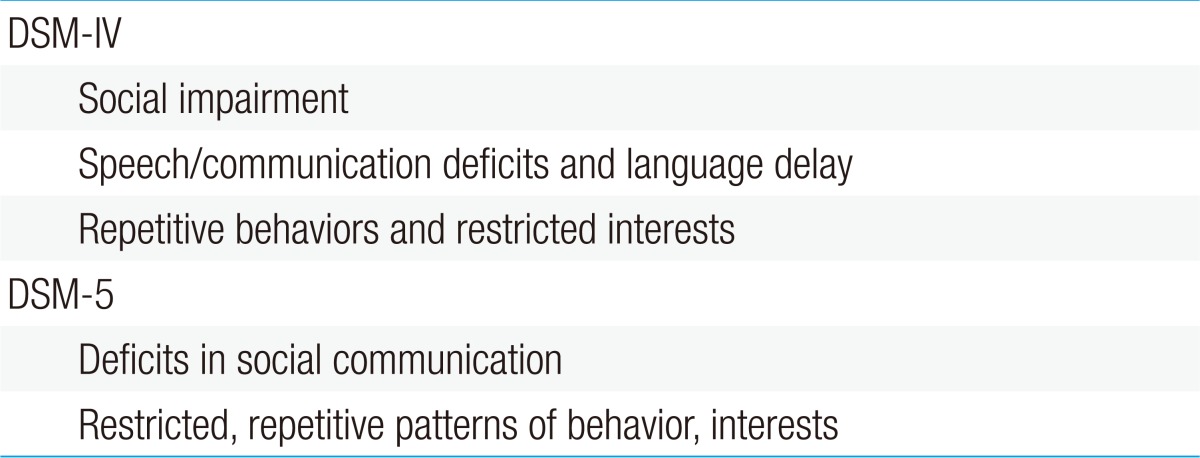
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, fifth edition.
Table 2. Categories of autism spectrum disorders.
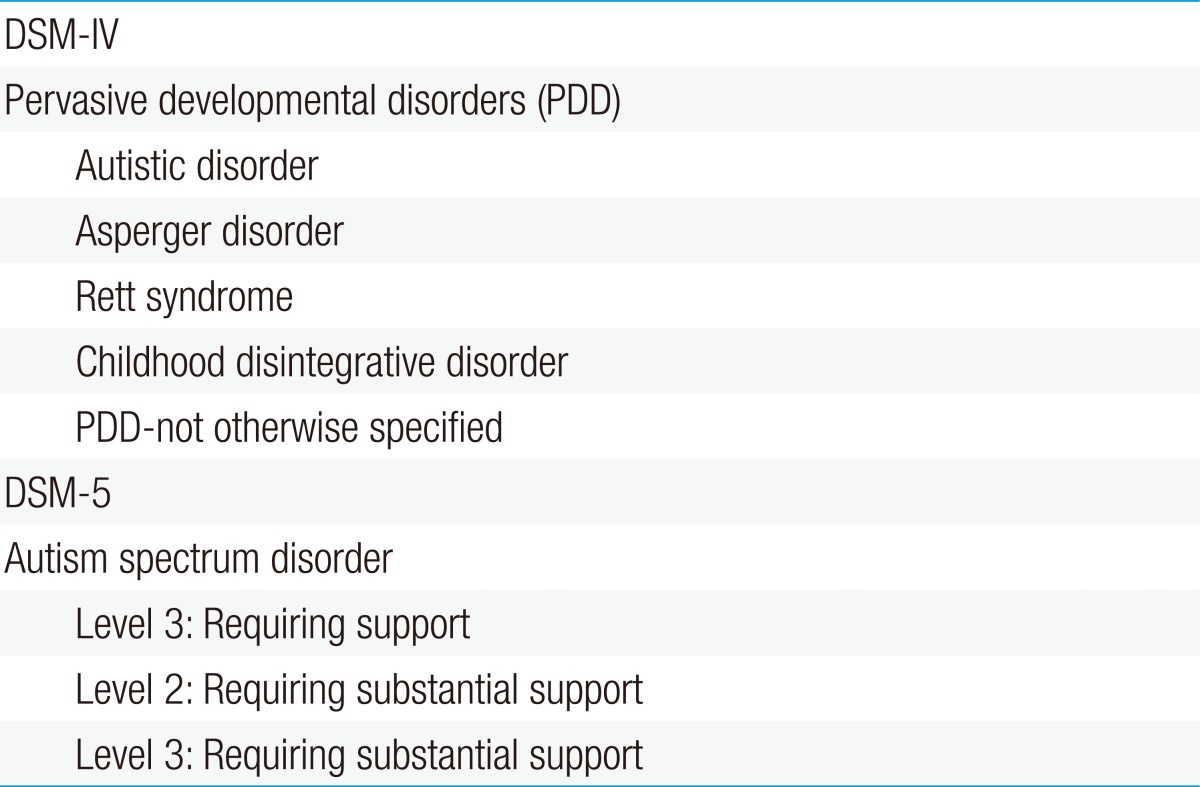
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, fifth edition.
Epidemiology
The prevalence of autism has increased dramatically, and it has been currently recognized as one of the most common developmental disorders. For many years after autism was first described in the 1940s, its prevalence was considered to be two to four cases per 10,000 children5).
Based on the most recent United States diagnostic survey reported by parents, the prevalence of ASD was as high as 11 per 1,0006). A number of factors have contributed to this increase. The diagnostic criteria for ASDs have been broadened; the concept of autism is now defined as an autistic disorder plus the broader ASDs, including Asperger syndrome and PDD-NOS. There is also a codiagnosis with known medical disorders such as fragile X syndrome, Tourette syndrome, and Down syndrome. Additionally, the growing public awareness among parents and teachers in developing countries has led to earlier diagnoses. Other factors include the increased availability of services and the ability to diagnose children at younger ages7,8).
Pathophysiology and etiology
1. Genetics
The known single-gene defects and the diagnosed medical conditions account for about 10% of the cases of autism9). Between 21% and 50% of the boys with fragile X syndrome are on the autistic spectrum10), and 0%-6% of the autism populations have fragile X syndrome11).
The prevalence rates of ASD in tuberous sclerosis complex (TSC) consistently range from 24% to 60%10). The incidence of autistic individuals with TSC complex has been estimated to be between 0.4% and 4% in epidemiologic studies12).
Copy number variants (CNVs) are DNA segments ranging in size from 50 base pairs to several megabases among individuals due to deletion, insertion, inversion, duplication, or complex recombination13). Recently, it has been demonstrated that the CNV location and its functional relevance may play a more crucial role than the mean CNV number and size.
Recently, two large datasets have been discovered: heterogeneous de novo copy-number variants collectively affecting several loci and presumably accounting for 5%-8% of the cases of simplex forms of ASD14). The network-based functional analysis of these rare CNVs confirms the involvement of these loci in synapse development, axon targeting, and neuron motility15).
Several neuroligins and SHANK and neurexin genes encoding proteins crucial to synapse formation, maturation, and stabilization have been found to host mutations responsible for behavioral phenotypes, including autism16). At the extracellular level, postsynaptic neuroligins interact with presynaptic or neurexins stimulating the formation of the presynaptic bouton17). At the intracellular level, neuroligins associate with postsynaptic scaffolding proteins such as SHANK318). Neuroligins are encoded by the NLGN1, NLGN2, NLGN3, NLGN4X, and NLGN5 genes. Among them, only the NLGN3, NLGN4, and NLGN4Y genes have been found to harbor mutations that could possibly cause autism.
Three members of the SHANK gene family (SHANK1, SHANK2, and SHANK3), which encode the scaffolding proteins are required for the proper formation and function of neuronal synapses. Recently, SHANK2 mutations have also been reported in both ASD and intellectual disability19).
The third crucial protein in the autism-related synaptic network is neurexins, encoded by the three highly conserved genes: NRXN1, NRXN2, and NRXN3.
The MECP2 gene is important for the correct brain function and development: loss of MECP2 has been shown to delay neuronal maturation and synaptogenesis, and MECP2 de novo loss-of-function mutations cause Rett syndrome in approximately 70% of the affected females, while they are generally found to be lethal in males20).
Children with idiopathic autism often display minor facial dysmorphisms and abnormal head/body growth rates21,22). Macrocephaly is seen in approximately 20% of children with autism22). The Hox genes play a crucial role during embryonic patterning and organogenesis. The phosphatase and tensin homolog (PTEN) gene located in chromosome 10q23, harbors mutations associated with a broad spectrum of disorders, including Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, Proteus syndrome, and Lhermitte-Duclos disease23).
PTEN is a tumor suppressor gene that favors cell cycle arrest in G1 and apoptosis. Genetic syndromes linked to PTEN germline haploinsufficiency are often associated with autism or mental retardation24).
The eukaryotic translation initiation factor 4E gene (EIF4E, located on chr. 4q21-q25) plays a pivotal role in protein translation downstream of mammalian target of rapamycin. Recently, a balanced translocation disrupting the EIF4E locus was found in a boy with ASD demonstrating regression of language and social interactions at 2 years of age25).
Recent genetic evidence proposes that at least some ASD cases may result from abnormal Ca2+ homeostasis during neurodevelopment26). Moreover, several genetic studies have found autism-related genes encoding proteins either directly or indirectly, controlling intracellular Ca2+ levels or regulated by cytosolic Ca2+ transients. The biochemical parameters linked to the mitochondrial function are frequently abnormal in autism27). It is only in rare instances that mutations in mitochondrial DNA or in nuclear DNA heavily involved in the mitochondrial function explain the disease. Children with mitochondrial disease thus, represent a small percentage (<1%) of all autistic patients. In reality, mitochondrial ASD forms are rare, as mitochondrial dysfunction appears to be secondary in most patients-i.e., downstream of other pathophysiological abnormalities such as excessive oxidative stress28).
2. Environmental factors
The expression of the autism gene may be influenced by environmental factors29). According to the epigenetic theory, environmental factors may modulate the already existing genetic factors responsible for the manifestation of ASDs in individual children. The increasing age of mothers is a risk factor of ASD, both independently and in combination with the increasing age of fathers30).
There may also be mutagens in the environment, such as mercury, cadmium, nickel, trichloroethylene, and vinyl chloride. The factors associated with vitamin D deficiency may cause mutations, as vitamin D contributes to the repair of DNA damage31).
3. Brain imaging
Ninety percent of children with autism had an above average brain volume at 2-4 years old and 37% had developmental macrocephaly, defined as a brain volume exceeding 2 standard deviations above the mean normal for that particular age32). This finding reveals an ongoing postnatal process that primarily affects the interhemispheric and cortical connections.
The results of the recent functional magnetic resonance imaging studies point to changes in the activation and synchronization of cortical networks. The functional connectivity of the networks is lowered, leading to deficits in language, social cognition, motor planning, and perception33).
4. Neuropathology
Kemper and Bauman34) described three major findings in children with autism: (1) curtailment of the normal development of the forebrain neurons, which were smaller and more densely distributed than normal; (2) an apparent congenital decrease in the number of Purkinje cells; and (3) age-related changes in cell size and in the number of neurons in the diagonal band of Broca, the cerebellar nuclei, and the inferior olive.
Clinical features
All individuals on the autistic spectrum demonstrate impairments in three symptom domains: reciprocal social interactions, verbal and nonverbal communication, and restricted and repetitive behaviors or interests. The cognitive function of ASD patients can range from profound mental retardation to the superior range on the conventional intelligence quotient tests.
1. Impairment of social interactions
ASD infants may or may not cuddle, or may even stiffen when held, and often do not look at the person or smile when interacting socially. Older children often do not point things out or use eye contact to share the pleasure of seeing something with another person, which is called joint attention or social referencing. Deficits in joint attention appear to be among the primary distinguishing characteristics of children with ASD35).
Children with autism may appear to ignore a familiar or unfamiliar person because of a lack of social interest. They may also have no age-appropriate friends and may prefer to play alone.
2. Impairment of communication
The expressive language function across the autistic spectrum ranges from complete mutism to verbal fluency. In early infancy, some children with ASD do not babble or use any other communicative vocalization, and are therefore, described as "quiet babies." Moreover, they cannot compensate for this language deficit with facial expressions or gestures. A hallmark of autistic speech is immediate or delayed echolalia. Some children with autism do not use toys, animals, or dolls in pretend play appropriately. In addition, they show aberrant play skills such as little symbolic play, ritualistic rigidity, and a preoccupation with specific parts of toys.
3. Restricted, repetitive, and stereotyped patterns of behavior and interests
Children with ASD can demonstrate atypical behaviors in a variety of areas, including peculiar mannerisms, unusual attachments to objects, obsessions, compulsions, self-injurious behaviors, and stereotypies. Children with autism ask the same question repeatedly, regardless of the reply that is given or engage in highly repetitive, perseverative play. They are also preoccupied with highly unusual special interests.
Many children with autism are preoccupied with consistency and prediction in their home and school environments or routines. Many of them demonstrate the classic behavior of lining up toys, videotapes, or other favored objects.
Diagnostic evaluation and screening
ASD can be reliably diagnosed in children as young as 2 years old and early intervention is beneficial36). The average age of diagnosis, however, is reported to be 3-6 years37). The American Academy of Pediatrics Council of Children with Disabilities recently published a set of guidelines on the identification and management of children with ASD38).
According to the screening algorithm of ASD, the patient at a preventive care visit or at an extra visit for an autism-related concern should identify the risk factors, such as a sibling with ASD, parental concern for ASD, other caregiver concern, or pediatrician concern, and each risk factor has a score of 138).
If a patient has a score of 2 or higher, there should be parental education, comprehensive ASD evaluation, early intervention/early childhood education services, audiologic evaluation, and a scheduled follow-up visit. If a patient has a score of 1, the intervention choice will depend on the child's age. If the patient is at least 18 months old, he/she has to take the ASD-specific screening test. After the test, if the result is positive following the two-score process and if the patient has no risk factor, ASD-specific screening will be recommended only if the visit is the 18 or 24-month preventive care visit.
1. Instruments for ASD screening
The Checklist for Autism in Toddlers, developed in Great Britain, is the most popular tool for screening 18- to 24-month-old children39). The Modified Checklist for Autism in Toddlers relies only on the parent's report40). The Screening Tool for Autism in Two-year-olds consists of interactive items administered by a clinician to a 24- to 35-month-old child41).
2. Instruments for ASD diagnosis
The Childhood Autism Rating Scale is a clinician-rated diagnostic instrument for use with children older than 2 years42).
The Autism Diagnostic Interview, Revised, a structured interview for parents, and the Autism Diagnostic Observation Schedule are considered as gold standards for the diagnosis of autism43).
Neurologic evaluation
1. Neurologic examination
Most investigators report that a small proportion of children with autism have remarkable macrocephaly. The abnormalities in the neurologic examination may include hypotonia, which was observed in children with autism. Hand or finger stereotypical movement, body rocking, and unusual posturing are reported in 37%-95% of autistic individuals and these symptoms often manifest during the preschool years36).
2. Evaluation of hearing
Many children diagnosed with autism are first described by their parents as acting "as if they are deaf." Audiologic evaluation or brainstem auditory-evoked potential testing should be performed in all children with autism so that if indicated, appropriate referrals can be made for aural habilitation38).
3. Electroencephalography
There is no sufficient evidence for or against the use of routine electroencephalogram (EEG) screening in ASD patients. A recent review found that epileptiform EEG abnormalities were present in 10.3%-72.4% of the patients and subclinical abnormalities in 6.1%-31% of the patients44).
4. Neuroimaging studies
Routine neuroimaging to evaluate a child with autism and macrocephaly is not warranted unless evidence of lateralizing signs is found in the neurologic examination.
5. Coexistent medical conditions
Children with ASD have several coexistent medical conditions such as gastrointestinal problems, sleep disturbance, epilepsy, and congenital blindness.
Treatment
1. Pharmacologic therapy
The goal of pharmacotherapy for children with autism is to alleviate the symptoms and specific behaviors. The target symptoms include sleep problems, anxiety, repetitive motor behaviors, obsessive-compulsive symptoms, impulsivity, depression, mood swings, agitation, hyperactivity, aggression, and self-injurious behavior.
Although there are no medications currently that directly impact cognitive impairment, controlling these symptoms should allow the child to maximize the benefits of educational and behavioral therapy that is directed more towards the core symptoms.
1) Neuroleptic agents
Risperidone and aripiprazole have been approved by the U.S. Federal Drug Administration for the treatment of irritability, such as aggression, self-injurious behavior, temper tantrums, and mood swings, in school-age children and adolescents with autistic disorders.
2) Serotonin reuptake inhibitors
The symptoms causing major impairment in autism, such as anxiety and repetitive and ritualized behaviors, can disrupt learning. Due to the effectiveness of serotonin reuptake inhibitors in alleviating the anxiety and obsessive-compulsive symptoms, and due to the finding that serotonin system abnormalities exist in individuals with autism45), there has been a considerable surge in treating disruptive behaviors in autism with these agents.
3) Stimulants and drugs for treating hyperactivity
Hyperactivity is an important target symptom that can be potentially alleviated with psychostimulant medication46).
4) Antiepileptic drugs
In open-label studies, levetiracetam and divalproex sodium appeared to be well tolerated and successful in alleviating repetitive behavior, impulsivity, and mood stability47,48).
2. Educational and behavioral interventions
The prioritization of interventions should focus on six specific areas: functional spontaneous communication, social instruction delivered throughout the day in various settings, play skills, cognitive development, proactive approaches to problem behaviors, and functional academics.
Methods based on applied behavior analysis (ABA) for teaching skills and facilitating more appropriate and adaptive behaviors have been tested extensively for their effectiveness in children and adults with autism and other developmental disabilities49). In the most rigorously designed studies of intensive early intervention programs based on ABA, efficacy was demonstrated at the group level, but the response was variable.
Conclusions
For patients with developmental delays, especially patients with language delays, a differential diagnosis for ASD must be conducted when they visit a pediatric clinic. In ASD patients, appropriate behavioral therapy and rehabilitation treatment significantly affect the prognoses, and hence, it is important to make an early diagnosis and to treat the disorder at an early stage.
Therefore, pediatricians must be able to recognize the signs and symptoms of ASD and must be attentive to their presence in order to make an early diagnosis and be able to treat the disorder at an early stage.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Eun BL, Kim SW, Kim YK, Kim JW, Moon JS, Park SK, et al. Overview of the national health screening program for infant and children. Korean J Pediatr. 2008;51:225–232. [Google Scholar]
- 2.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 3.Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. pp. 50–59. [Google Scholar]
- 5.Wing L, Potter D. The epidemiology of autistic spectrum disorders: is the prevalence rising? Ment Retard Dev Disabil Res Rev. 2002;8:151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- 6.Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 7.Nassar N, Dixon G, Bourke J, Bower C, Glasson E, de Klerk N, et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol. 2009;38:1245–1254. doi: 10.1093/ije/dyp260. [DOI] [PubMed] [Google Scholar]
- 8.Parner ET, Schendel DE, Thorsen P. Autism prevalence trends over time in Denmark: changes in prevalence and age at diagnosis. Arch Pediatr Adolesc Med. 2008;162:1150–1156. doi: 10.1001/archpedi.162.12.1150. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- 10.Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53:852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- 11.Fombonne E, Du Mazaubrun C, Cans C, Grandjean H. Autism and associated medical disorders in a French epidemiological survey. J Am Acad Child Adolesc Psychiatry. 1997;36:1561–1569. doi: 10.1016/S0890-8567(09)66566-7. [DOI] [PubMed] [Google Scholar]
- 12.Chudley AE, Gutierrez E, Jocelyn LJ, Chodirker BN. Outcomes of genetic evaluation in children with pervasive developmental disorder. J Dev Behav Pediatr. 1998;19:321–325. doi: 10.1097/00004703-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, et al. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 21.Tripi G, Roux S, Canziani T, Bonnet Brilhault F, Barthelemy C, Canziani F. Minor physical anomalies in children with autism spectrum disorder. Early Hum Dev. 2008;84:217–223. doi: 10.1016/j.earlhumdev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Sacco R, Militerni R, Frolli A, Bravaccio C, Gritti A, Elia M, et al. Clinical, morphological, and biochemical correlates of head circumference in autism. Biol Psychiatry. 2007;62:1038–1047. doi: 10.1016/j.biopsych.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet. 2008;16:1289–1300. doi: 10.1038/ejhg.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet. 2001;105:521–524. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- 25.Neves-Pereira M, Muller B, Massie D, Williams JH, O'Brien PC, Hughes A, et al. Deregulation of EIF4E: a novel mechanism for autism. J Med Genet. 2009;46:759–765. doi: 10.1136/jmg.2009.066852. [DOI] [PubMed] [Google Scholar]
- 26.Krey JF, Dolmetsch RE. Molecular mechanisms of autism: a possible role for Ca2+ signaling. Curr Opin Neurobiol. 2007;17:112–119. doi: 10.1016/j.conb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Giulivi C, Zhang YF, Omanska-Klusek A, Ross-Inta C, Wong S, Hertz-Picciotto I, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304:2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmieri L, Persico AM. Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim Biophys Acta. 2010;1797:1130–1137. doi: 10.1016/j.bbabio.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Lawler CP, Croen LA, Grether JK, Van de Water J. Identifying environmental contributions to autism: provocative clues and false leads. Ment Retard Dev Disabil Res Rev. 2004;10:292–302. doi: 10.1002/mrdd.20043. [DOI] [PubMed] [Google Scholar]
- 30.Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168:1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney DK, Barch DH, Chayka B, Napoleon S, Munir KM. Environmental risk factors for autism: do they help cause de novo genetic mutations that contribute to the disorder? Med Hypotheses. 2010;74:102–106. doi: 10.1016/j.mehy.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 33.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Mundy P, Markus J. On the nature of communication and language impairment in autism. Ment Retard Dev Disabil Res Rev. 1997;3:343–349. [Google Scholar]
- 36.Lord C. Follow-up of two-year-olds referred for possible autism. J Child Psychol Psychiatry. 1995;36:1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- 37.Howlin P, Moore A. Diagnosis of autism: a survey of over 1200 patients in the UK. Autism. 1997;1:135–162. [Google Scholar]
- 38.Johnson CP, Myers SM American Academy of Pediatrics Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 39.Baird G, Charman T, Cox A, Baron-Cohen S, Swettenham J, Wheelwright S, et al. Current topic: Screening and surveillance for autism and pervasive developmental disorders. Arch Dis Child. 2001;84:468–475. doi: 10.1136/adc.84.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robins DL, Dumont-Mathieu TM. Early screening for autism spectrum disorders: update on the modified checklist for autism in toddlers and other measures. J Dev Behav Pediatr. 2006;27(2 Suppl):S111–S119. doi: 10.1097/00004703-200604002-00009. [DOI] [PubMed] [Google Scholar]
- 41.Stone WL, Coonrod EE, Ousley OY. Brief report: screening tool for autism in two-year-olds (STAT): development and preliminary data. J Autism Dev Disord. 2000;30:607–612. doi: 10.1023/a:1005647629002. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski C, Green JA, Barton ML, Fein D. Using the childhood autism rating scale to diagnose autism spectrum disorders. J Autism Dev Disord. 2010;40:787–799. doi: 10.1007/s10803-009-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 44.Kagan-Kushnir T, Roberts SW, Snead OC., 3rd Screening electroencephalograms in autism spectrum disorders: evidence-based guideline. J Child Neurol. 2005;20:197–206. doi: 10.1177/08830738050200030601. [DOI] [PubMed] [Google Scholar]
- 45.Chandana SR, Behen ME, Juhasz C, Muzik O, Rothermel RD, Mangner TJ, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30:245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- 47.Rugino TA, Samsock TC. Levetiracetam in autistic children: an open-label study. J Dev Behav Pediatr. 2002;23:225–230. doi: 10.1097/00004703-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Hollander E, Dolgoff-Kaspar R, Cartwright C, Rawitt R, Novotny S. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry. 2001;62:530–534. doi: 10.4088/jcp.v62n07a05. [DOI] [PubMed] [Google Scholar]
- 49.Dunlap G, Fox L. A demonstration of behavioral support for young children with autism. J Posit Behav Interv. 1999;1:77–87. [Google Scholar]


